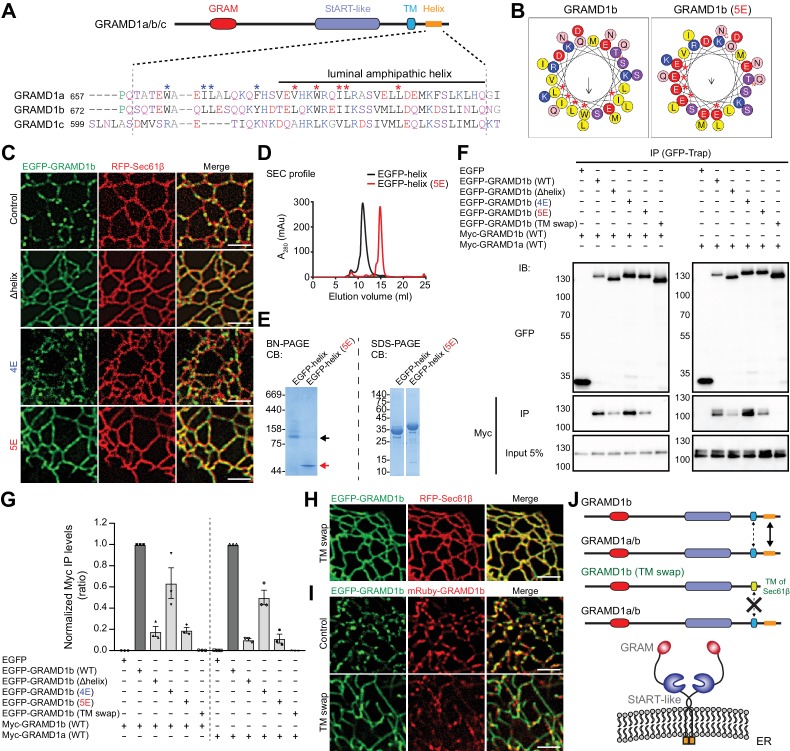
Figure 2. Luminal helix and transmembrane domain of GRAMD1b are important for homo- and heteromeric interaction.
(A) Sequence alignment of the luminal region of GRAMD1s. This region is predicted by Phyre2 to contain an amphipathic helix (Kelley et al., 2015) as indicated. Blue and red asterisks mark hydrophobic amino acid residues that are partially conserved in GRAMD1s. The shared identities of the amino acid sequences of the amphipathic helices predicted by BLAST analysis were: 75% (GRAMD1a vs. GRAMD1b); 75% (GRAMD1a vs. GRAMD1c); and 80% (GRAMD1b vs. GRAMD1c). The effects of the mutations of these residues to glutamic acid (4E in the case of blue marks; 5E in the case of red marks) were tested in GRAMD1b. Black, red, blue, and pink/purple colors denote hydrophobic, acidic, basic, and hydrophilic amino acid residues, respectively. (B) Predicted luminal amphipathic helix region of wild-type GRAMD1b (left panel) and that with L693E, W696E, I699E, I700E and L707E (5E) mutations (right panel) are shown as helical wheel representations. Predictions were made with the Heliquest server (Gautier et al., 2008). (C) Confocal images of live COS-7 cells expressing RFP–Sec61β and EGFP fusions of various GRAMD1b constructs [control, wild-type GRAMD1b; Δhelix, GRAMD1b lacking the predicted luminal amphipathic helix; 4E, GRAMD1b with 4E mutations in the luminal region (W678E, L681E, L682E, Y688E); 5E, GRAMD1b with 5E mutations in the predicted luminal amphipathic helix]. Note the reduced formation of GRAMD1b patches in Δhelix and 5E mutants but not in the 4E mutant. Scale bars, 2 µm. (D) Overlay of the size exclusion chromatography (SEC) profiles of the recombinant EGFP-tagged luminal helix region of wild-type GRAMD1b (EGFP–helix) and EGFP–helix with the 5E mutations [EGFP–helix (5E)]. Note the difference in elution volumes, indicating the formation of complexes mediated by the wild-type luminal helix. (E) Blue native (BN)-PAGE analysis (left panel) and SDS-PAGE analysis (right panel) of SEC-purified EGFP–helix and EGFP–helix (5E). Black and red arrows indicate the major bands for EGFP–helix and EGFP–helix 5E, respectively. Note the difference in their migration pattern in BN-PAGE. CB, Colloidal blue staining. (F) Extracts of HeLa cells transfected with the constructs as indicated were subjected to anti-GFP immunoprecipitation (IP) and then processed for SDS-PAGE and immunoblotting (IB) with anti-GFP and anti-Myc antibodies. Inputs are 5% of the total cell lysates. Note that the interaction of GRAMD1b or GRAMD1a is much reduced in GRAMD1b Δhelix or 5E mutants and abolished in the GRAMD1b (TM swap) mutant (GRAMD1b with its transmembrane domain and luminal region replaced with those of Sec61β) when compared to the levels of interactions seen in cells with wild-type GRAMD1b. This reduction is smaller in the GRAMD1b 4E mutant. (G) Quantification of the co-immunoprecipitation experiments shown in (F). The ratio of the band intensity of the co-immunoprecipitated Myc–GRAMD1b (left) or Myc–GRAMD1a (right) over that of the indicated immunoprecipitated EGFP-tagged proteins were calculated. The values were then normalized by the ratio of the band intensity of Myc–GRAMD1b over that of EGFP–GRAMD1b (WT) (left) or by the ratio of the band intensity of Myc–GRAMD1a over that of EGFP–GRAMD1b (WT) (right) [mean ± SEM, n = 3 IPs for each sample]. (H) Confocal images of a live COS-7 cell expressing RFP-Sec61β and EGFP-tagged GRAMD1b (TM swap). Scale bars, 2 µm. (I) Confocal images of live COS-7 cells expressing mRuby–GRAMD1b and EGFP fusions of GRAMD1b constructs [Control, wild-type GRAMD1b; TM swap, GRAMD1b (TM swap)]. Note the abolished formation of GRAMD1b patches in TM swap mutants. Scale bars, 2 µm. (J) Model of the homo- and heteromeric interactions of GRAMD1a/b. Their complex formation is facilitated primarily by their luminal amphipathic helices and additionally mediated by their transmembrane domains. These regions are important for the ability of GRAMD1s to form complexes and patches on the tubular ER network.