Fig. 5. Protective efficacy of the pVAX-tpaNS1 vaccine.
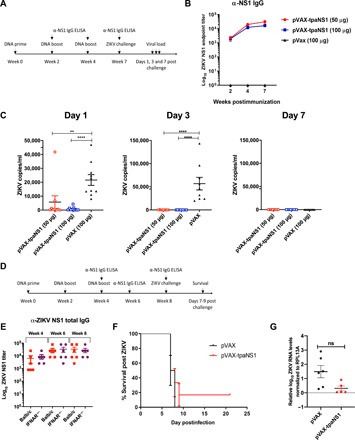
(A) Timeline of vaccination, challenge, and viral load determinations. Female Balb/c mice (n = 10) were immunized intradermally three times with either 50 or 100 μg of the pVAX-tpaNS1 DNA vaccine. Control pVAX mice received three 100-μg doses. (B) anti-NS1 antibody titer as measured by ELISA. (C) Mice were challenged 3 weeks after the last dose by the intravenous route with 200 PFU of ZIKVPRVABC59. Serum ZIKV VL on days 1, 3, and 7 after challenge are shown (**P < 0.005, ****P < 0.0001, Kruskal-Wallis H test). (D) Timeline of vaccination, determination of NS1 antibody responses and lethal challenge of female IFNAR−/− mice. (E) Female IFNAR−/− (n = 6) and Balb/c (n = 7) mice were immunized intradermally three times with 50 μg of pVAX-tpaNS1 or pVAX control DNA vaccine. Anti-ZIKV NS1 antibody titers were determined at weeks 4, 6, and 8 of the immunization schedule by NS1 ELISA and compared with antibody titers in Balb/c mice. (F) Survival of pVAX-tpaNS1– or pVAX-vaccinated IFNAR−/− mice after subcutaneous challenge with 103 CCID50 of ZIKVMR766. Mice were euthanized when ethically defined endpoints had been reached. (G) Viremia in pVAX-tpaNS1– or pVAX-vaccinated IFNAR−/− mice after challenge with ZIKVMR766. Limit of detection 2 log10CCID50/ml. Error bars represent SEM.
