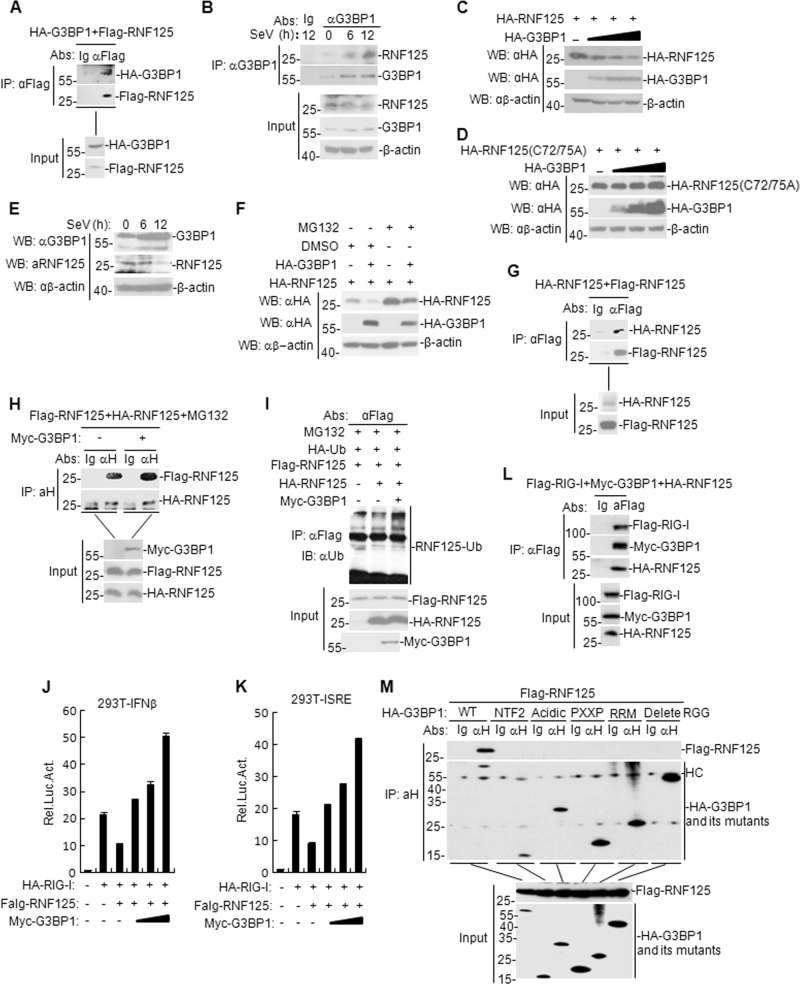
Fig. 8. G3BP1 promotes degradation of RNF125 via its auto-ubiquitination.
a Interaction between G3BP1 and RNF125 in the mammalian overexpression system. HEK293T cells were transfected with the indicated plasmids (5 μg of each) for 24 h. Co-IP and immunoblotting were performed with the indicated antibodies. b Endogenous G3BP1 interacted with RNF125 in HEK293T cells. HEK293T cells (5 × 107) were untreated or infected with SeV for the indicated time. Co-IP and immunoblotting experiments were performed with the indicated antibodies. c, d Effects of G3BP1 on the expression of RNF125 or RNF125 (C72/75 A) mutant were evaluated. HEK293T cells were transfected with HA-G3BP1 (0, 0.5, 1.5, and 3 μg) and HA-RNF125 or HA-RNF125 (C72/75 A) plasmids (2 μg) for 24 h. Then the cell lysates were analyzed by immunoblotting with the indicated antibodies. e Effects of SeV infection on the expression of endogenous G3BP1 and RNF125 in HEK293T cells. HEK293T cells were uninfected or infected with SeV for the indicated time. The cell lysates were analyzed by immunoblotting with the indicated antibodies. f Effects of MG132 on G3BP1-mediated destabilization of RNF125. HEK293T cells (4 × 105) were transfected with the indicated plasmids for 18 h and then the cells were treated with DMSO or MG132 for 6 h before immunoblotting analysis. g Interaction between RNF125 and RNF125 in the mammalian overexpression system. HEK293T cells were transfected with the indicated plasmids (5 μg of each). Co-IP and immunoblotting were performed with the indicated antibodies. h Effects of G3BP1 on the interaction between RNF125 and RNF125. The experiments were similarly to those described in g. i Effects of G3BP1 on the ubiquitination of RNF125. HEK293T cells (2 × 106) were transfected with the indicated plasmids for 18 h and then treated with MG132 for 6 h. Co-IP and immunoblotting were performed with the indicated antibodies. j, k Effects of G3BP1 overexpression on RNF125-mediated RIG-I activation were assessed. HEK293T cells (1 × 105) were transfected with the IFN-β reporter, ISRE (0.1 μg), HA-RIG-1 (100 ng), Flag-RNF125 (100 ng) or G3BP1 (0, 100, 200, and 400 ng) expression plasmids for 24 h before luciferase assays were performed. The experiment was repeated in triplicates. l Interaction between G3BP1, RIG-I, and RNF125 in HEK293T cells. HEK293T cells were transfected with the indicated plasmids for 24 h. Co-immunoprecipitation and immunoblotting analysis were performed with the indicated antibodies. m Interaction between G3BP1, G3BP1 mutants, and RNF125. The experiments were similarly to those described in l. Data are mean ± SD of three independent experiments. Co-IP Co-immunoprecipitation, EV, empty vector, Luc luciferase, αH anti-HA tag, HC heavy chain.