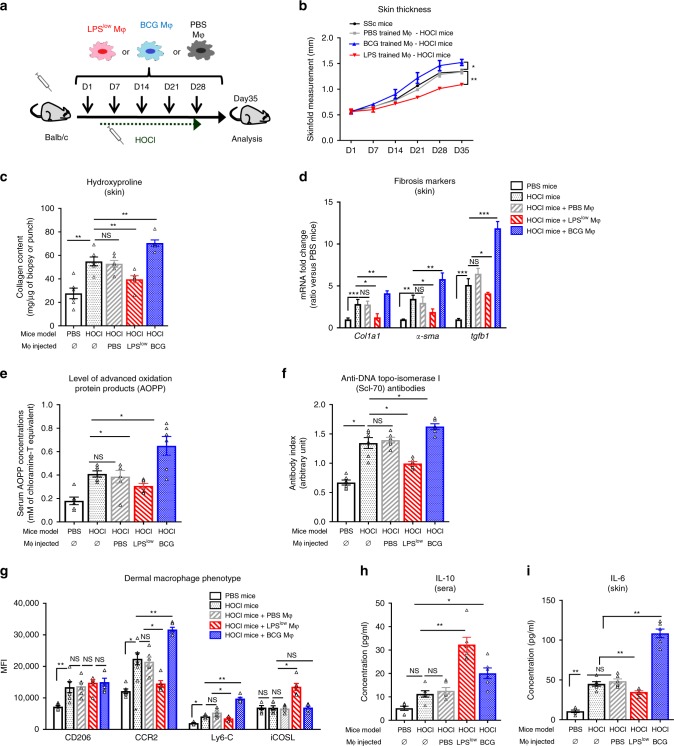
Fig. 5. Adoptive transfer of LPSlow trained macrophages ameliorates SSc.
a Experimental setup of therapeutic intraperitoneal injection of in vitro trained macrophages to HOCl-mice (n = 6 in each group). b Evolution of skin fold thickness in millimeters from days 1 to 35, measured weekly. c Collagen content in skin (Hydroxyproline dosage, mg/punch of biopsy). Each box represents mean ± SEM from n = 6 biologically independent mice. d Col1a1, α-sma, and Tgf-β mRNA levels in skin. Results were expressed as fold increase versus the PBS control group, derived from n = 6 biologically independent mice (Unpaired T-test). e Concentrations of anti-DNA topo-isomerase I (Scl70) antibodies in the sera from mice (Arbitrary Unit). Each box represents mean ± SEM from n = 6 biologically independent mice. f Concentration of advanced oxidation protein products (AOPP) in the sera from mice (mM of chloramine T equivalent). g Flow cytometric analysis of dermal macrophage phenotype at day 35. Dermal cell suspensions were prepared as for the previous experiments. Macrophages were gated on CD11b+F4/80 +cells. Data represent the MFI and SEM from n = 6 biologically independent mice. h, i ELISA assessment of IL-6 production by skin-derived cells (h) and seric IL-10 concentration (i). Each box represents the mean concentration (pg/ml) ± SEM from n = 6 biologically independent mice. Statistics are shown between PBS and HOCl-mice first and then macrophage injected HOCl-mice (PBS, LPSlow and BCG) versus un-injected HOCl-mice. The ANOVA test with Bonferroni correction was used to detect significant differences between the groups (unless stated). Mϕ: Macrophages. NS: Not significant; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001. Source data are provided as a Source Data file.