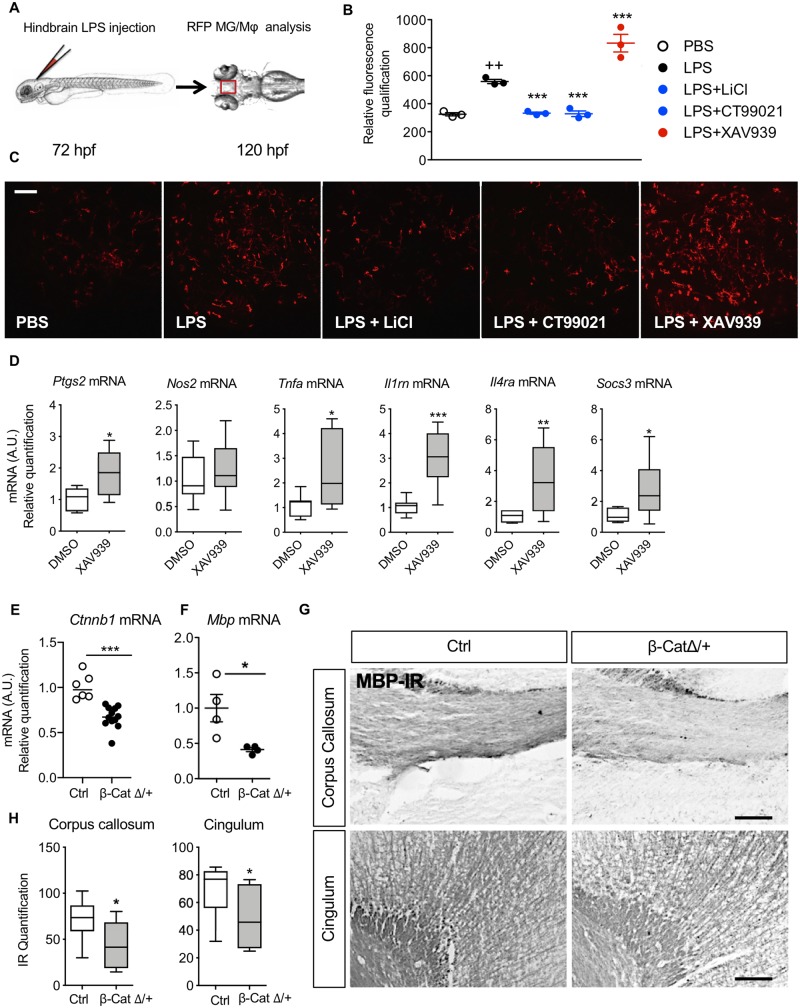
Figure 4.
Wnt/β-catenin pathway regulates MG/Mϕ activation and hypomyelination in vivo. Wnt signalling was modulated in vivo with pharmacological techniques in zebrafish (A–C) and mice (D) and using gene knockout in mice (E–G). (A) Location of LPS microinjection (5 ng/injection) into the zebrafish hindbrain at 72 hpf in pu1::Gal4-UAS::TagRFP morpholinos followed by analysis at 120 hpf. (B) Scatter plot of quantification of RFP labelled microglia and (C) representative images of RFP microglia in these zebrafish brains after LPS injection in the presence or not in their growth solution of Wnt/β-catenin pathway activators LiCl (80 mM) and CT99021 (3 μM) or Wnt inhibitor XAV939 (5 μM) (mean ± SEM, one-way ANOVA with post hoc Newman-Keuls’s test, **P < 0.01, ***P < 0.001). Scale bar = 40 μm. (D) Analysis of the phenotype of CD11B+ cells isolated from P1 mice injected intracerebroventricularly with XAV939 (0.5 nmol) alone (no intraperitoneal IL-1β), demonstrating increased MG/Mϕ activation with Wnt agonism. Min to max box and whiskers plots of quantification of Nos2, Ptgs2, Tnfa, Il1rn, Socs3 and Il4ra mRNA by RT-qPCR. mRNA levels are presented as a fold-change relative to PBS/DMSO group (Student’s t-test, *P < 0.05, **P < 0.01 and ***P < 0.001 n = 7–10/group. (E and F) β-Catenin deficit in microglia driven by β-cateninflox/+/LysMCre/+ (β-cateninΔ/+) induces hypomyelination. Scatter plots of quantification by RT-qPCR of (E) Ctnnb1 mRNA deficit in CD11B+ MG/Mϕ from P10 β-cateninΔ/+ mice (n = 6–12/group) and (F) Mbp mRNA deficit in the anterior brain at P10 in β-cateninΔ/+ mice. mRNA levels are presented as a fold-change relative to control (Ctrl) mice (β-catenin+/+/LysMCre/+) (n = 4/group) (mean, Mann-Whitney test, *P < 0.05, ***P < 0.01). (H) Min to max box and whiskers plot of grey density level of MBP immunoreactivity (MBP-IR) in the corpus callosum and cingulum at P30 relative to control mice. (Mann-Whitney test, *P < 0.05, n = 6/group and illustrated by representative images of MBP-IR in G. Scale bar = 40 μm.