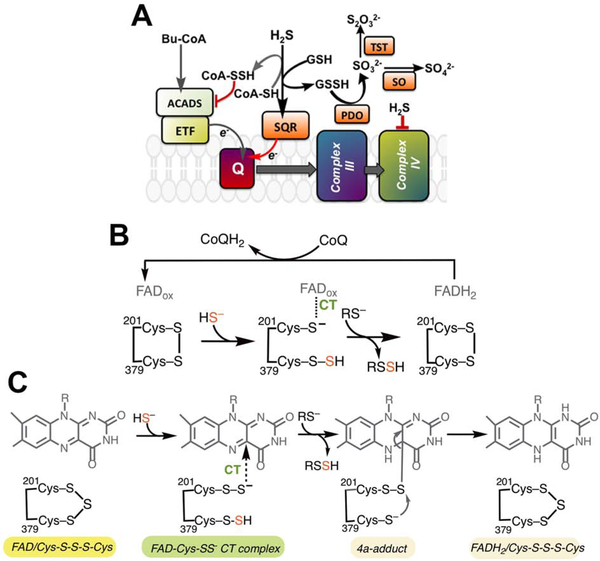
Figure 1. Intersection between sulfide and butyrate oxidation pathways and the proposed mechanisms for the SQR reaction.
(A) The canonical SQR reaction utilizes GSH as an acceptor to generate GSSH, with electrons driven into the CoQ10 (Q) pool. ACADS via the electron transferring flavoprotein (ETF), also drives electrons into the Q pool via oxidation of butyrate. We hypothesized that SQR utilizes CoA as an alternate acceptor generating CoA-SSH, which inhibits ACADS and blocks electron flow from butyrate oxidation. PDO (or ETHE1), TST and SO represent persulfide dioxygenase, rhodanese and sulfite oxidase, respectively in the mitochondrial sulfide oxidation pathway. (B) The previously proposed SQR reaction mechanism in which the active site cysteines in the resting enzyme form a disulfide. Cys201 participates in a CT complex with FAD while Cys379 is the sulfane sulfur carrier. (C) A proposal for the SQR-catalyzed oxidative half reaction, informed by our crystal structures, invokes a trisulfide configuration of the active site cysteines. Sulfide addition leads to persulfides on both cysteines. 201Cys-SS− forms a CT complex with FAD while 379Cys-SSH serves as the sulfur donor.