Fig. 1.
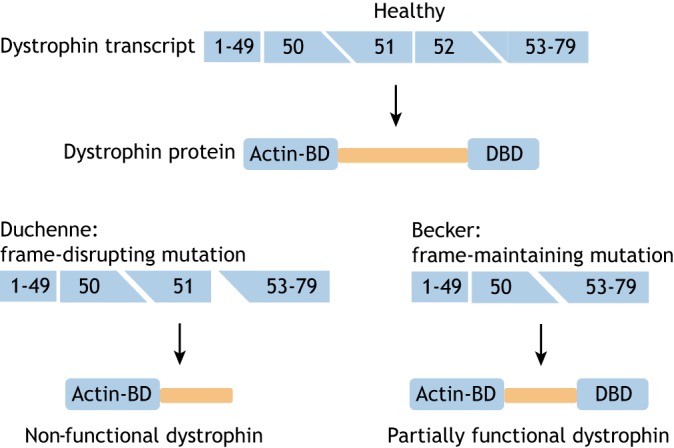
Dystrophin mutations underlie both Duchenne and Becker muscular dystrophy. The DMD gene encodes the dystrophin protein, which links the actin-cytoskeleton to the extracellular matrix with its actin binding domain (Actin-BD) and dystroglycan binding domain (DBD), respectively (upper panel). In Duchenne patients, mutations, generally deletions involving one or more exons, disrupt the reading frame. In the example in the bottom left panel, an exon 52 deletion causes a frameshift and premature truncation of protein translation, and a non-functional dystrophin. In Becker patients, deletions maintain the reading frame. In the example in the bottom right panel a deletion of exon 51-52 does not disrupt the reading frame, thus allowing the production of an internally deleted dystrophin that contains both crucial domains and that, consequently, is partially functional.
