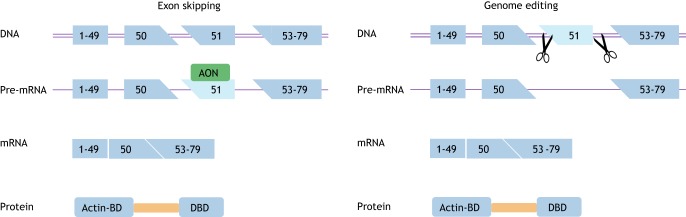
Fig. 2.
Schematic depiction of therapeutic exon skipping and genome editing approaches for Duchenne muscular dystrophy. Exon skipping (left panel) interferes in the pre-mRNA splicing process using antisense oligonucleotides (AON) that target a specific exon (exon 51 in this example). Thus the target exon is hidden from the splicing machinery and ‘skipped’ from the mature mRNA. This enlarges the deletion, but restores the reading frame, thus allowing the production of an internally deleted Becker-like dystrophin. Genome editing (right panel) acts on the DNA level, using guide RNAs that guide the Cas9 enzymes (scissors) to specific locations in the gene. This will result in double-stranded DNA breaks, which are repaired by non-homologous end joining in postmitotic cells (such as skeletal muscle fibers), leading to a larger deletion. Consequently, all transcripts produced have an in-frame deletion, thus allowing the production of internally deleted Becker-like dystrophin. Actin-BD, actin binding domain; DBD, dystroglycan binding domain.