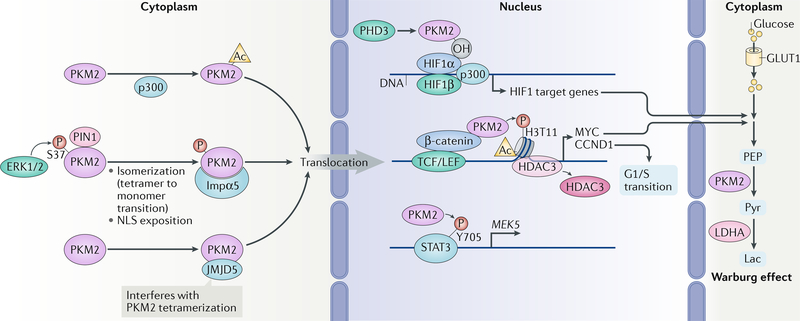
Fig. 4 |. The roles of nuclear PKM2 in gene expression.
The nuclear translocation of pyruvate kinase M2 isoform (PKM2) is regulated by several mechanisms. ERK1 and ERK2 phosphorylate PKM2, which promotes its isomerization (transition from tetramer to monomer) mediated by peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), resulting in the exposition of nuclear localization signal (NLS) of PKM2 and its interaction with importin α5 (Impα5). Nuclear translocation of PKM2 is also promoted by acetylation (Ac) mediated by histone acetyltransferase p300 and by the interaction of PKM2 with Jumonji domain-containing protein 5 (JMJD5). In the nucleus, PKM2 interacts with β-catenin and binds to β-catenin-regulated promoters, where it phosphorylates histone H3 at T11 and subsequently removes histone deacetylase 3 (HDAC3) to promote histone H3 acetylation and expression of genes regulating the Warburg effect and cell cycle progression. PKM2 also directly increases the transcriptional activity of signal transducer and activator of transcription 3 (STAT3) by phosphorylation (P) of STAT3. PKM2 hydroxylated by prolyl hydroxylase 3 (PHD3) binds to hypoxia-inducible factor 1α (HIF1α) to promote recruitment of HIF1α and p300 to the promoters of HIF1α-regulated genes for expression. CCND1, G1/S-specific cyclin D1; GLUT1, glucose transporter type 1, erythrocyte/brain (also known as SLC2A1); Lac, lactate; LDHA, l-lactate dehydrogenase A chain; LEF, lymphoid enhancer factor; MYC, MYC proto-oncogene protein; OH, hydroxylation; PEP, 2-phosphoenolpyruvate; Pyr, pyruvate; TCF, T cell factor.