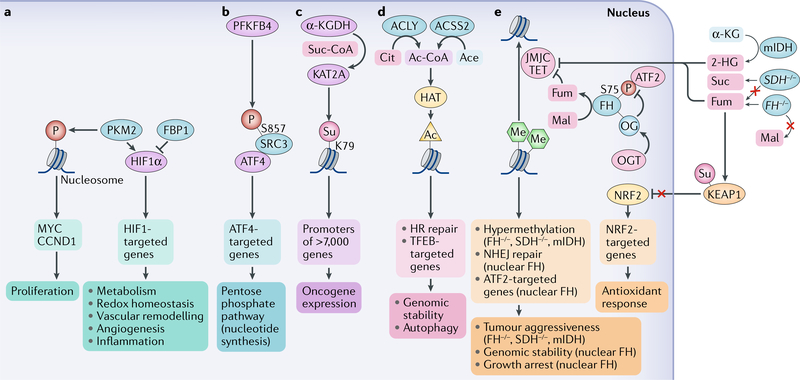
Fig. 6 |. Association of metabolites and metabolic enzymes with cancer development.
a | Nuclear pyruvate kinase M2 isoform (PKM2) promotes gene expression through phosphorylation (P) of histones or regulation of transcription factors, such as hypoxia-inducible factor 1α (HIF1α). HIF1α activity can also be upregulated by fructose-1,6-bisphosphatase 1 (FBP1) deficiency. b | 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) promotes gene expression through phosphorylation of steroid receptor co-activator protein 3 (SRC3) at S857. This phosphorylation increases the interaction between SRC3 and activating transcription factor 4 (ATF4), leading to gene expression. c | In the nucleus, α-ketoglutarate dehydrogenase (α-KGDH) interacts with histone acetyltransferase KAT2A and locally supplies succinyl-CoA (Suc-CoA). This high local concentration of Suc-CoA fuels succinylation (Su) of K79 in histones in the promoter regions of more than 7,000 genes, promoting oncogene expression. d | ATP citrate (Cit) synthase (ACLY) and nuclear acetyl-CoA (Ac-CoA) synthetase short-chain family member 2 (ACSS2) generate local Ac-CoA, which promotes histone acetylation (Ac) by histone acetyltransferases (HATs) and regulates homologous recombination (HR)-dependent DNA repair and the expression of lysosome and autophagy genes under the control of transcription factor EB (TFEB). e | α-KG dioxygenases, including the ten-eleven translocation (TET) and Jumonji C (JMJC) domain-containing demethylases are inhibited by high levels of succinate (Suc) induced by loss-of-function mutations in genes encoding succinate dehydrogenase (SDH−/−), by high levels of fumarate (Fum), which accumulate in the absence of fumarase (FH) activity in the tricarboxylic acid (TCA) cycle owing to loss-of-function mutations in the fumarase gene (FH−/−) as well as by aberrant production of 2-hydroxyglutarate (2-HG) induced by mutation of isocitrate dehydrogenases: cytoplasmic (mIDH1) and mitochondrial (mIDH2). This inhibition contributes to the hypermethylation phenotype and aggressiveness of tumours with SDH, FH or IDH1/2 mutations. High levels of Fum also increase the transcriptional activity of nuclear factor erythroid 2-related factor 2 (NRF2) and subsequent antioxidant response by promoting succinylation (Su) and, in turn, inhibition of Kelch-like ECH-associated protein 1 (KEAP1).Wild-type fumarase can be targeted to the nucleus, where it contributes to local production of Fum from malate (Mal) and inhibition of demethylases, which locally increases histone methylation (Me) to support nonhomologous end joining (NHEJ) DNA repair. In addition, wild-type fumarase as a tumour suppressor can be negatively regulated by O-GlcNAcylation (OG) mediated by O-GlcNAc transferase (OGT), which interferes with fumarase phosphorylation and the interaction of fumarase with ATF2, leading to suppression of ATF2-regulated cell growth arrest genes. Ace, acetate; CCND1, G1/S-specific cyclin D1.