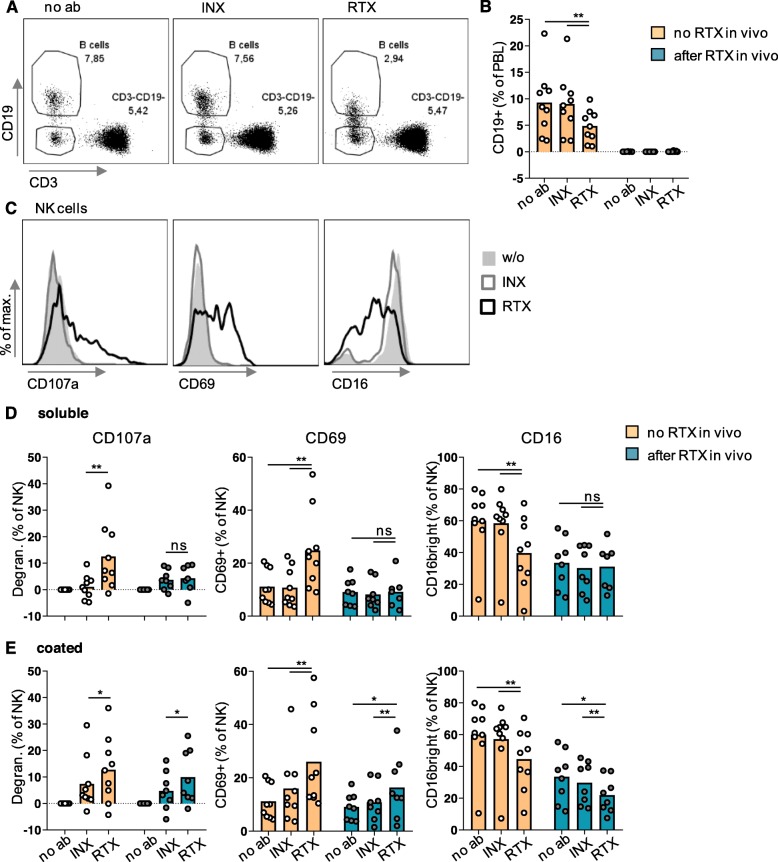
Fig. 3.
Simultaneously to B cell reduction, Rituximab led to degranulation and activation of GPA NK cells—provided that rituximab was bound to a surface. PBMCs from the 17 GPA patients shown in Fig. 2 were divided into two groups: nine were rituximab-naive and eight rituximab-experienced. PBMCs were cultured overnight with or without soluble or coated rituximab (RTX), infliximab (INX), or anti-CD16 antibody in the presence of a fluorochrome-labeled anti-CD107a antibody. PBMCs were then stained and analyzed as described in Fig. 2. a, b B cells were defined as life CD3-CD19+ lymphocytes (PBL). a Exemplary dot plots originating from the same rituximab-naive patient shown in Fig. 2a. As a fraction of B cells seemed to lose CD19 upon incubation with rituximab and as the viability dye cannot exclude early apoptotic cells, we provide data in Additional file 2 showing that the loss of CD19 is associated with cell death. b Percentages of B cells as a function of in vivo and in vitro treatment with rituximab. c-e NK cells were defined as viable CD3-CD19-CD56+ PBL. c Exemplary histograms originating from PBMCs from the patient after incubation with soluble antibodies; w/o, without antibody. d-e Degranulation was defined as [(% of CD107a+ NK cells after culture with therapeutic antibody) − (% of CD107a+ NK cells after culture without antibody)]. Degranulation, CD69 expression, and percentage of CD16bright cells were analyzed. Statistical analyses for comparison of groups of biological interest were performed using Wilcoxon test and are indicated in the graphs. Bars represent means