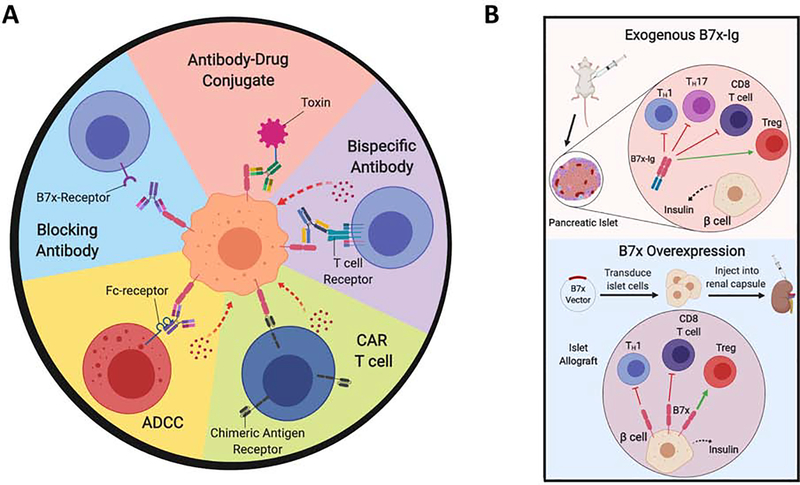
Figure 4. Therapeutics Targeting the B7x Pathway.
(A) Several strategies are being pursued to target B7x in human cancers, and have demonstrated efficacy in pre-clinical murine models. Blocking the interaction of B7x with its receptor on immune cells with anti-B7x monoclonal antibodies eliminates this mechanism of immune escape. Further, anti-B7x antibodies can be used to target B7x-expressing tumor cells for cytotoxic or immune-mediated killing. Anti-B7x antibodies can mediate antibody-dependent cellular cytotoxicity through Fc-dependent killing. Further, anti-B7x/anti-CD3 bispecific antibodies crosslink B7x to the T cell receptor on T cells, allowing effector T cells to kill target tumor cells. Anti-B7x antibodies can be crosslinked with toxins such as MMAE to kill tumor cells. B7x-expressing tumor cells can also be targeted by cellular therapies such as chimeric antigen receptor CAR-T cells, where T cells are engineered to target B7x on tumor cells, and thus mediate immune killing. (B) Two strategies have been used to activate the B7x pathway for the treatment of autoimmune disease such as Type 1 Diabetes. The B7x-Ig fusion protein of the extracellular region of B7x and the immunoglobulin-Fc domain can be administered systemically, which binds to the B7x-receptor on T cells and thereby induces systemic immunosuppression. B7x-Ig inhibits the effector functions of effector CD4 (Th1, Th17 cells) and CD8 T cells, and promotes immunosuppressive Tregs. Alternatively, the B7x gene can be genetically overexpressed in a target tissue. For example, a B7x-overexpression vector is transduced into pancreatic islet cells, upon which they are transplanted into the host organism. The renal capsule is a clinically relevant site to engraft the islet cells. B7x-overexpression in these cells suppresses autoreactive T cells, promotes Tregs, and thereby prolongs survival of the islet allografts.