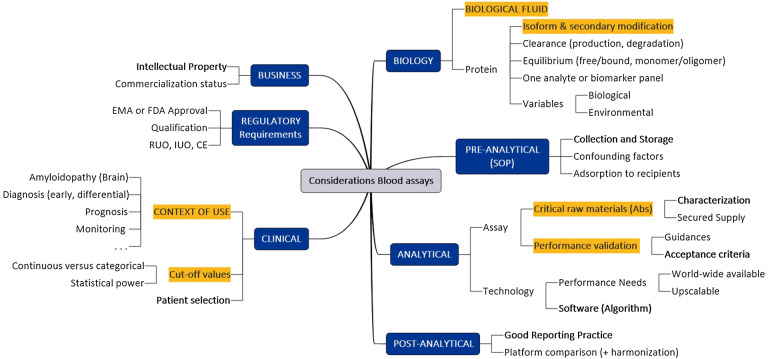
Fig. 1.
Factors to be considered during implementation of blood-based biomarker analysis. Analysis of considerations to be taken into account for protein analysis in blood samples, divided in function of the topic (blue boxes). Yellow boxes have a direct relationship with the context of use (COU). Bold/underlined words are considered as more critical for the future development of blood-based biomarker panels. EMA European Medicines Agency, FDA The Food and Drug Administration, IUO for investigation use only, IVD in vitro diagnostics, RUO research use only, SOP standard operating procedure