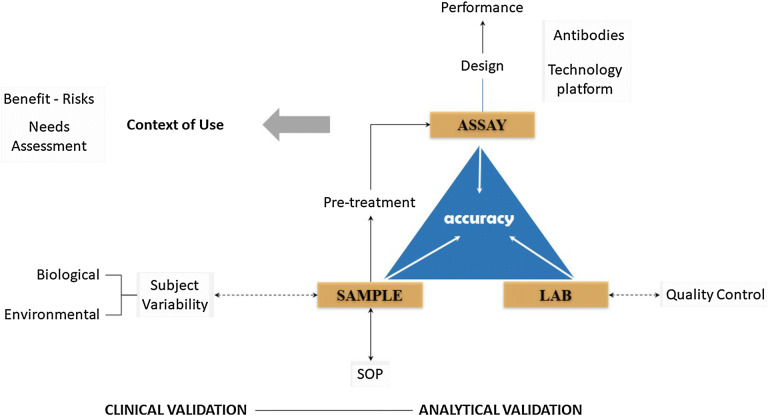
Fig. 2.
Precision qualified assays (PQAs). The general concept of assay validation. Clinical and analytical validation for a specific COU are distinct processes, but closely dependent on one another. PQAs will provide a solution by better combining clearly defined analytical performance requirements of an assay (e.g., selectivity, specificity, accuracy, linearity/parallelism) with the observed effects in a patient. PQAs means that results of the analytical performance studies are interpreted in view of their effects for individual patient management. SOP standard operating procedure