Table 1.
Screening of reaction conditionsa.
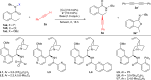 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | 1 | [Cu] | Base | L* | Solvent | Yield (%, 3a, 3a′, 3a″)b | Ee (%)c |
| 1 | 1aa | CuI | Cs2CO3 | L1 | Dichloromethane (DCM) | –d, 0, 0 | – |
| 2 | 1aa | CuI | Cs2CO3 | L2 | DCM | –d, 0, 0 | – |
| 3 | 1aa | CuI | Cs2CO3 | L3 | DCM | <5, 0, 0 | 6 |
| 4 | 1aa | CuI | Cs2CO3 | L4 | DCM | 8, 0, 0 | 74 |
| 5 | 1aa | CuI | Cs2CO3 | L5 | DCM | 53, 0, –d | 68 |
| 6 | 1aa | CuI | Cs2CO3 | L6 | DCM | 15, 0, 0 | 51 |
| 7 | 1ab | CuI | Cs2CO3 | L5 | DCM | –d, 48, 0 | – |
| 8 | 1ac | CuI | Cs2CO3 | L5 | DCM | 0, 0, –d | – |
| 9 | 1aa | CuI | Cs2CO3 | L5 | 1,2-Dichloroethane | 50, 0, –d | 76 |
| 10 | 1aa | CuI | Cs2CO3 | L5 | Benzene | 77, 0, 5 | 64 |
| 11 | 1aa | CuI | Cs2CO3 | L5 | EtOAc | 92, 0, 0 | 91 |
| 12 | 1aa | CuI | Cs2CO3 | L5 | THF | 88, 0, 0 | 94 |
| 13 | 1aa | CuBr | Cs2CO3 | L5 | THF | 64, 0, 0 | 93 |
| 14 | 1aa | CuTc | Cs2CO3 | L5 | THF | 91, 0, 0 | 92 |
| 15 | 1aa | CuOAc | Cs2CO3 | L5 | THF | 80, 0, 0, | 94 |
| 16 | 1aa | CuI | Na2CO3 | L5 | THF | <5, 0, 81 | 85 |
| 17 | 1aa | CuI | K2CO3 | L5 | THF | 27, 0, 62 | 93 |
| 18 | 1aa | CuI | KOtBu | L5 | THF | 71, 0, 26 | 93 |
| 19e | 1aa | CuI | Cs2CO3 | L5 | THF | 66, 0, 0 | 94 |
| 20f | 1aa | CuI | Cs2CO3 | L5 | THF | 31, 0, 0 | 92 |
| 21 | 1aa | CuI | Cs2CO3 | – | THF | 0, 0, 0 | – |
| 22g | 1aa | CuI | Cs2CO3 | – | THF | 0, 0, 0 | – |
| 23g | 1aa | CuI | Cs2CO3 | L5 | THF | 0, 0, 0 | – |
aReaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), [Cu] (10 mol%), L* (10 mol%), and base (0.1 mmol) in dry solvent (1.2 mL) at room temperature (rt) for 16 h
bYield based on 1H NMR analysis of the crude product using CH2Br2 as an internal standard
cEe values based on HPLC analysis
dA trace amount of product
eCuI (5 mol%), L* (5 mol%) for 24 h
fCuI (2 mol%), L* (2 mol%) for 36 h
gWithout 2a
