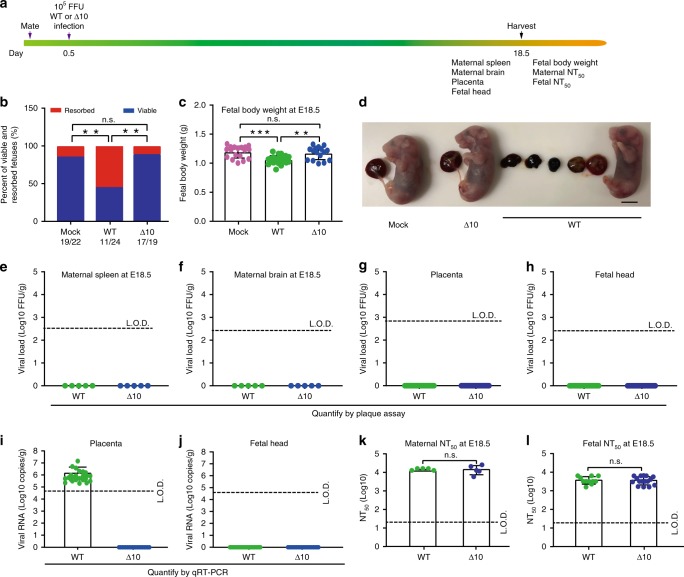
Fig. 1. The safety of 3′UTR-Δ10-LAV in pregnant A129 mice when vaccinated at E0.5.
a Experimental scheme. Ten- to twelve-week-old pregnant mice were subcutaneously infected with 105 FFU WT ZIKV (n = 5) and 3′UTR-Δ10-LAV (Δ10; n = 5) at E0.5. Maternal and fetal tissues were harvested and analyzed at E18.5. b The percentage of fetuses that were resorbed during pregnancy (n = 22 for mock; n = 24 for WT ZIKV; n = 19 for Δ10 vaccine; chi-square test [**p < 0.01]). The numbers of normal fetuses and total fetuses are presented below each group. c Fetal body weight at E18.5 for placebo, WT ZIKV, and Δ10 groups. Asterisks indicate significant differences as analyzed by one-way ANOVA. **p < 0.01, ***p < 0.001, p > 0.5 non-significant (n.s.). d Representative images of fetuses collected at E18.5 from placebo, Δ10, and WT ZIKV groups. Scale bar, 5 mm. Viral loads quantified by plaque assay at E18.5 are presented for maternal spleen (e), brain (f), placenta (g), and fetal head (h). Viral RNA measured by quantitative RT-PCR (qRT-PCR) at E18.5 are presented for placenta (i) and fetal head (j). k Maternal neutralizing antibody titers from WT ZIKV- and Δ10-infected pregnant mice at E18.5 with duplicate technical replicates. n.s. non-significant (Mann–Whitney test). l Neutralizing antibody titers in fetuses from WT ZIKV- and Δ10-infected dams at E18.5 with duplicate technical replicates. L.O.D. limit of detection, n.s. non-significant (Mann–Whitney test). Error bars represent standard deviations. Source data are provided as a Source Data file.