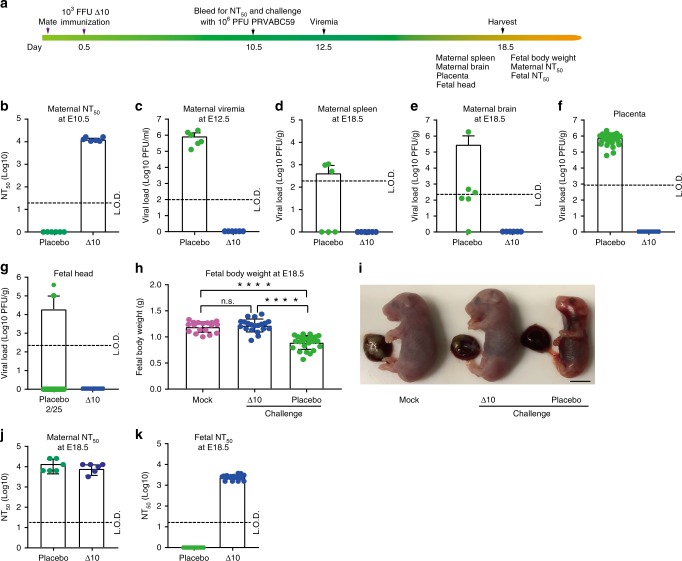
Fig. 2. The efficacy of 3′UTR-Δ10-LAV in pregnant A129 mice when immunized at E0.5.
a Experimental scheme. At E0.5, ten- to twelve-week-old pregnant mice were subcutaneously immunized with 103 FFU 3′UTR-Δ10-LAV (Δ10, n = 6) or PBS placebo (n = 6). At E10.5, the pregnant mice were bled for NT50 measurement and subcutaneously challenged with 106 PFU of ZIKV PRVABC59. Viremia was quantified by at E12.5. Maternal and fetal tissues were harvested for analysis at E18.5. b Neutralizing antibody titers from PBS- or Δ10-immunized pregnant mice at E10.5. c Viremia at E12.5. Viral loads at E18.5 are presented for maternal spleen (d), brain (e), placenta (f), and fetal head (g). (h) Fetal body weights at E18.5 from three experimental groups: non-immunized, un-challenge (mock; left panel); Δ10-vaccinated, challenged (middle panel); and PBS-immunized, challenged (right panel). Asterisks indicate significant differences (one-way ANOVA). ****p < 0.0001; non-significant (n.s) p > 0.5. Because the experiments in Figs. 1 and 2 were performed at the same time, the mock group in (h) is the same as the mock group in Fig. 1c; this reduced the number of animal use. (i) Representative images of fetuses collected at E18.5 from the three groups in (h). Scale bar, 5 mm. (j) Maternal neutralizing antibody titers from pregnant mice at E18.5. (k) Fetal neutralizing antibody titers from fetuses at E18.5. Duplicate technical replicates were performed for (j) and (k). Error bars represent standard deviations. Source data are provided as a Source Data file.