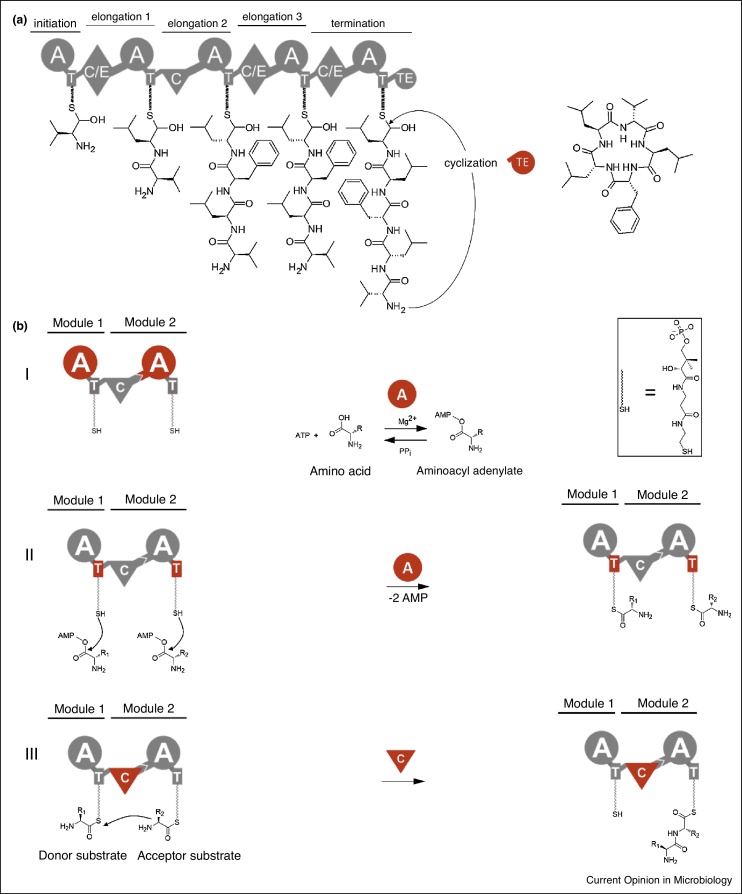
Figure 1.
Introduction to modular non-ribosomal peptide synthetases.
(a) The modular architecture of NRPSs, exemplified by the GameXPeptide-Synthetase (GxpS). A NRPS module is defined as the catalytic unit responsible for the incorporation of one-specific building block into the growing peptide chain and associated functional group modifications. Enzymatic activities of a canonical module reside in the adenylation (A), thiolation (T), and condensation (C) domains. (b) Firstly, amino acids (AAs) are activated through the activity of an A-domain (I). The energy derived from ATP hydrolysis is used to form an aminoacyl-adenylate intermediate. Then the AA is loaded onto the thiol of the pantetheine cofactor of the T domain (II). Consecutively activated AA on T domains are then joined by a C-domain that catalyses peptide-bond formation and transfers the upstream AA or peptide to the downstream substrate (III). Finally, most NRPS termination modules harbour a TE (thioesterase) domain, usually responsible for the release of linear (transfer to a water molecule), cyclic or branched cyclic peptides (amide or ester linkage). In addition, a cyclization (Cy) domain instead of a C-domain or a terminal condensation (Cterm) domain in place of a TE-domain can be present. Modification domains include epimerization (E), N-methylation (MT) or oxidation (Ox) domains.