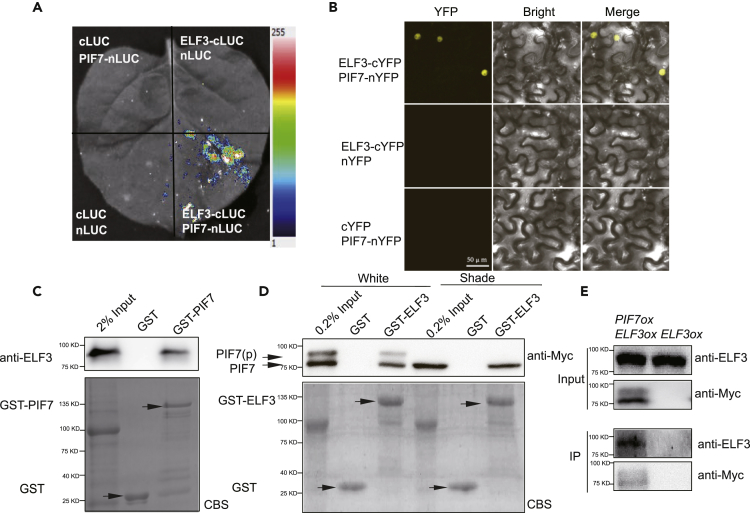
Figure 3.
Interaction between PIF7 and ELF3
(A) Interactions between PIF7 and ELF3 were detected with a bimolecular fluorescence complementation assay based on firefly luciferase (LUC). The N- and C-terminal halves of LUC were fused to PIF7 and ELF3, respectively. Constructs were coexpressed in tobacco leaf cells. Luciferin was infiltrated before LUC activity was monitored.
(B) Bimolecular fluorescence complementation analysis of the interaction between PIF7 and ELF3 in tobacco leaf cells. The C-terminal half of YFP was fused to ELF3, and the N-terminal half of YFP was fused to PIF7. The constructs were cotransformed into tobacco leaf cells, and fluorescence images were obtained by confocal microscopy.
(C) The interaction between ELF3 extracted from plants and purified GST-PIF7 from E. coli in the GST pull-down assay. Top panel: the pull-down fractions and inputs were analyzed by western blots using anti-ELF3 antibodies. Bottom panel: Coomassie brilliant blue R250-stained (CBS) proteins on an SDS-PAGE gel are shown.
(D) The interaction between purified GST-fused ELF3 from E. coli and total protein extracts from plants overexpressing PIF7-Flash grown under white light conditions or treated with 1 h of shade, as indicated by GST pull-down assays. Top panel: the pull-down fractions and inputs were analyzed by western blots using anti-Myc antibodies. Bottom panel: Coomassie brilliant blue R250-stained (CBS) proteins on an SDS-PAGE gel are shown.
(E) Coimmunoprecipitation analysis of the interaction between PIF7 and ELF3. Anti-FLAG sepharose beads were used to precipitate PIF7-Flash from PIF7ox plants. Western blot was performed using anti-Myc and anti-ELF3 antibodies as indicated.