STRUCTURED ABSTRACT:
Background:
Chronicity of depression among people living with HIV (PLWH) is associated with poorer viral suppression and mortality risk. The extent to which suicidal ideation (SI) and other baseline characteristics predict a prolonged duration of depressive illness among PLWH is not known but could help identify PLWH most at risk.
Methods:
Data were drawn from a sample of 1002 depressed PLWH engaged in primary care at a metropolitan HIV clinic from 2007-2018, representing 2,569 person-years. Depression characteristics were derived from the Patient Health Questionnaire 9 (PHQ-9), administered during routine screening. Other characteristics were derived from clinic data. Unadjusted and covariate-adjusted survival analyses compared the time to depression remission between depressed participants with and without SI at their initial screening.
Results:
At baseline, 38.4% of depressed PLWH endorsed SI. Depressed PLWH with SI took significantly longer to achieve remission from depression than those without SI. The association appeared to be mediated by depression symptom severity. When adjusted for age, depression diagnosis, any recent drug use, and depression symptom severity, baseline SI no longer predicted remission hazard.
Limitations:
Participants were assessed for depression with variable frequency. The analysis assumed all patients received comparable treatment for their depression. Some variables were based on clinic measurements that may be subject to misclassification bias.
Conclusions:
These data suggest that depressed PLWH with SI are at risk for greater chronicity of depression because their depression is more severe. Accordingly, PLWH should be urgently referred to psychiatric care in the event of SI or severe depressive symptoms.
Keywords: AIDS, HIV, major depressive disorder, suicide, suicidality
BACKGROUND:
Depression is highly prevalent among people living with HIV (PLWH) (Badiee et al., 2012; Catalan et al., 2011; Nanni et al., 2015) and occurs at rates 2-4 times that in the general population (Bing et al., 2001; Ciesla and Roberts, 2001). Depressive symptoms are associated with poorer disease outcomes in PLWH (Friedman et al., 2015; Ickovics et al., 2001), lower adherence to antiretroviral medication, and poorer psychosocial functioning (Blashill et al., 2015; Friedman et al., 2015; Peltzer et al., 2015). Although depression treatment is associated with improvements in affective symptoms and adherence to antiretroviral treatment (Eshun-Wilson et al., 2018; Pence et al., 2015; Sin and DiMatteo, 2014), many PLWH with depression do not receive evidence-based treatment (Cholera et al., 2017; Pence et al., 2012). This treatment gap likely contributes to chronicity of depression in this population, a factor that has been associated with missed clinic appointments, poorer viral suppression, and mortality (Pence et al., 2018).
PLWH also experience higher rates of suicidal ideation (SI) than the general population and are at an increased risk of completing suicide, even in the era of highly active antiretroviral therapy (HAART) (Jia et al., 2012; Keiser et al., 2010; Kelly et al., 1998). In cross-sectional studies, SI or suicide risk among PLWH has been associated with comorbid psychiatric illness, unsuppressed viral load, and the presence of an AIDS-defining illness (Badiee et al., 2012; Jia et al., 2012; Kang et al., 2016; Keiser et al., 2010; López et al., 2018; Protopopescu et al., 2012).
Although SI and longer duration of depressive illness share an association with poorer HIV treatment outcomes, there are currently no studies examining the association between SI and depression chronicity among PLWH. Here we have applied longitudinal analytical methods to archived patient data from a large sample of PLWH engaged in primary care to compare length of time to remission of depression between depressed PLWH who do and do not have SI. We also examined the relative contribution of SI and other patient-level characteristics to length of time to remission of depression. Because urgent psychiatric treatment to address SI and factors associated with a prolonged course of depression may improve mental health and HIV outcomes, this investigation of the course of depressive illness among PLWH and the particular role of SI may help to identify those at risk for HIV-related morbidity and mortality.
METHODS:
Data were derived from the medical records and self-report assessments of PLWH receiving primary care at the HIV clinic of a metropolitan academic center. The clinic currently serves approximately 4,842 PLWH, 61% of whom completed self-report assessments known as Patient Reported Outcomes (PROs) as part of routine care. PROs were collected from participants at regular intervals, approximately every 3-6 months. Clinical information from the electronic medical record complemented the PROs and were abstracted to a central database without personally identifiable information. Data from this central database were used for this analysis. Our academic center’s Institutional Review Board exempted this study from review.
Analysis sample
The nine-item Patient Health Questionnaire (PHQ-9), a validated depression screening tool (Kroenke et al., 2001), was routinely collected during PROs beginning in November 2007. Participants were included if they had completed the PHQ-9 in at least two PROs between November 2007 and January 2018 and met the study cutoff for depression at their baseline PRO. Because including subscores of the PHQ-9 SI screening item (item 9) may introduce bias, we calculated a “PHQ-8” score by subtracting item 9 scores from the PHQ-9 total score. Participants were classified as depressed if their baseline PHQ-8 score was 10 or greater, which has been validated as a screening threshold for depression (Kroenke et al., 2009). Participants with a baseline PHQ-8 score less than 10, even if they reported SI, were excluded in order to evaluate the relationship of SI to depression chronicity. Participants were also excluded if the participant did not respond to the PHQ-9 at their baseline PRO or if the demographic variables of age or sex were missing.
Measures
Depression symptom severity, SI, and remission from depression
Depression symptom severity, SI, and remission from depression were captured by the PHQ-9. The PHQ-8 score at baseline was used to measure depression symptom severity. Participants were classified as having SI if they endorsed SI at their baseline assessment for at least “several days” over the preceding two weeks on item 9 of the PHQ-9 (“thoughts that you would be better off dead, or of hurting yourself in some way”) which has been associated with an increased risk of subsequent suicide attempt or death (Simon et al., 2013). Our outcome of interest, time to remission from depression, was defined as the time between the baseline PHQ-8 score and the first follow-up PHQ-8 with a score less than 5 (Kroenke et al., 2010), indicating the first recorded remission from their baseline depression.
Demographics and HIV risk behaviors
Demographics included age, sex, and race/ethnicity. Because the association between HIV and suicide was observed to be stronger before the introduction of HAART in a Swiss nationwide study (Jia et al., 2012), we controlled for cohort effects by calculating a dichotomous indicator for whether a participant was diagnosed with HIV in the pre-HAART or HAART era, defined as before or after January 1, 1997, respectively. We also extracted self-reported risk factors for HIV infection such as identifying as a man who has sex with men (MSM) or having a history of injection drug use (IDU), as both have been associated with a greater risk of suicide in PLWH (Carrieri et al., 2017; Keiseret al., 2010).
Diagnosis of psychiatric and substance use disorders
Mental and substance use disorders have been shown to increase the risk of subsequent suicide among PLWH (Jia et al., 2012). We used ICD-9 and/or ICD-10 codes derived from the electronic medical record to identify psychiatric and substance use disorders diagnosed at the time of, before, or up to seven days after each participant’s first PHQ-9 measurement. The seven-day post-PHQ window was intended to capture baseline variables recorded on dates immediately following the initial assessment, whether erroneously or due to an interrupted encounter. Psychiatric diagnoses were grouped into corresponding categories: “depression,” “bipolar disorder,” “anxiety,” “psychosis,” “PTSD,” and “adjustment disorder” (Supplemental Table S1-S2), and substance use disorder diagnoses were grouped by substance (alcohol, stimulants, opioids, sedatives, cannabis, other substances) and combined into an “any” substance use disorder category (Supplemental Table S3-S4).
Self-reported substance use
Published studies of PLWH identified associations between substance abuse and past suicidal ideation, plan, or attempt (Badiee et al., 2012; Carrieri et al., 2017). Patients reported drug use on PROs using the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Lifetime history of drug use was included in the analysis if participants reported it at the time of, before, or up to seven days after the baseline PHQ-9 (Humeniuk et al., 2008). Recent drug use was included if participants answered ‘yes’ to drug use in the last three months at any time from one year before the baseline PHQ-9 to seven days afterwards. Drugs were categorized as amphetamines, street opiates, cocaine or crack, and marijuana, with a composite ‘any drug use’ term for any use of the four. We likewise identified harmful alcohol use from the Alcohol Use Disorders Identification Test – Concise (AUDIT-C). An AUDIT-C score of greater than or equal to 4 for men and greater than or equal to 3 for women was considered positive (Bradley et al., 2007).
Psychiatric medication
Because persons with a lifetime suicide attempt were more likely to be prescribed psychiatric medications than non-attempters (Badiee et al., 2012), we extracted psychiatric medication prescription from the medical record to account for any confounding influence. Psychiatric medications were included if the participant had an active prescription at the time of their baseline PHQ-9. We categorized medications according to drug class: antidepressants, sedative/hypnotic medications including benzodiazepines, antipsychotics, and mood stabilizers (see Supplemental Table S5).
Antiretroviral medication
We extracted select antiretroviral medications (ARVs) if the patient had an active prescription for any ARV regimen containing the drugs dolutegravir, efavirenz, raltegravir, or rilpivirine at the time of their baseline PHQ-9. Dolutegravir, rilpivirine, and efavirenz were selected due to observational studies showing an association with neuropsychiatric adverse events (Fettiplace et al., 2017; Hoffmann et al., 2017), case reports of psychiatric symptoms (Freeman and Levenson, 2015; Kheloufi et al., 2015), or a evidence of an increased risk of subsequent suicidality (Mollan et al., 2014). Raltegravir, which is generally well tolerated, was selected as a control.
HIV viral load
We included detectable viral load in the analysis because it is associated with severe psychiatric events including suicide or suicide attempt (Protopopescu et al., 2012). We used the viral load result occurring closest to the baseline PHQ-9 and drawn within 90 days before or up to seven day after the baseline PHQ-9. We considered HIV viral load detectable at above a threshold of 400 copies/ml as this was the lowest-sensitivity threshold for detection among viral load tests administered to our sample.
Recent HIV diagnosis
A higher prevalence of SI has been observed immediately after HIV diagnosis, which then decreases over time (Perry et al., 1990). We accounted for adjustment to this life event by classifying patients who received their HIV diagnosis within 90 days of their baseline assessment to be recently diagnosed.
Prior psychiatric office visit
A patient was considered to have had prior psychiatric office visit if they had a completed visit with a psychiatric provider registered in the electronic medical record before their baseline PHQ-9.
Analysis
In descriptive analyses, distributions of demographic and clinical variables were compared between participants with and without SI using Pearson’s chi-square test for categorical variables, and independent-samples t-test for scalar variables. We compared the distribution of observations obtained using the Wilcoxon rank sum test.
We compared the time to depression remission between participants with and without SI at the time of their first PHQ screening by the Gehan-Breslow-Wilcoxon test, and by the unadjusted hazard ratio from the Cox proportional hazards model.
To compare the hazard of depression remission between the groups with and without SI at baseline, when adjusting for covariates of interest, we used a multi-predictor Cox proportional hazards model. First, we performed single-predictor Cox regression on all covariates of interest. The terms which were significant at p < 0.2 were considered as candidates for the multi-predictor model. Backward model selection was used to arrive at a final Cox proportional hazards model, including the SI variable and all covariates significant at p ≤ 0.05 in the step-wise procedure. For both the unadjusted and adjusted Cox proportional hazards models, we assessed predictive accuracy with Harrell’s C-statistic for Cox regression. We tested for proportionality of hazard using Schoenfeld residuals. To assess the relationship between depression severity and SI in the time to depression remission analysis, we performed a sensitivity analysis excluding baseline PHQ-8 score as a covariate of interest in model selection.
RESULTS:
Characteristics of Sample
A sample of 1002 participants met criteria for our primary analysis, representing 2,569 person years. Baseline characteristics of depressed PLWH with and without SI are described in Table 1. Participants were primarily white, men, and MSM. At baseline, 38.4% of depressed PLWH in the study endorsed SI. A significantly greater proportion of participants with SI carried a diagnosis of depression or had been seen by a psychiatrist prior to baseline. Likewise, patients with SI had a significantly higher baseline PHQ-8 score than the non-suicidal comparison group. A significantly greater number of PHQ-9 observations were available for participants with SI at baseline and for those who had attained remission.
TABLE 1.
Descriptive comparison of baseline characteristics in the sample of n = 1002 participants
| Characteristic | Depressed without SI | Depressed with SI | P value | ||
|---|---|---|---|---|---|
| N | Proportion of sample (%) |
N | Proportion of sample (%) |
||
| Total | 617 | 61.6% | 385 | 38.4% | |
| N | Proportion of subgroup (%) |
N | Proportion of subgroup (%) |
Chi-squared test | |
| Sex | 0.114 | ||||
| Female | 94 | 15.2% | 45 | 11.7% | |
| Male | 523 | 84.8% | 340 | 88.3% | |
| Race/ethnicity | 0.959 | ||||
| Non-Hispanic white | 320 | 51.9% | 195 | 50.6% | |
| Non-Hispanic black | 84 | 13.6% | 49 | 12.7% | |
| Hispanic | 174 | 28.2% | 117 | 30.4% | |
| Asian/PI | 17 | 2.8% | 11 | 2.9% | |
| Other | 22 | 3.6% | 13 | 3.4% | |
| HIV risk factor | |||||
| MSM | 448 | 72.6% | 294 | 76.4% | 0.187 |
| IDU | 80 | 13.0% | 55 | 14.3% | 0.552 |
| Heterosexual | 121 | 19.6% | 66 | 17.1% | 0.329 |
| Unknown/decline | 18 | 2.9% | 7 | 1.8% | 0.278 |
| Era of HIV diagnosis | 0. 230 | ||||
| Pre-HAART | 200 | 32.4% | 139 | 36.1% | |
| Post-HAART | 417 | 67.6% | 246 | 63.9% | |
| Viral load | 0.405 | ||||
| Detectable | 201 | 39.3% | 137 | 42.2% | |
| Undetectable | 311 | 60.7% | 188 | 57.8% | |
| Missing | 105 | 17.0% | 60 | 15.6% | 0.612 |
| Recent (3 mo.) HIV diagnosis | 87 | 14.1% | 46 | 11.9% | 0.329 |
| Recent/detectable* | 0.424 | ||||
| No/no | 301 | 58.8% | 185 | 56.9% | |
| No/yes | 134 | 26.2% | 98 | 30.2% | |
| Yes/no | 10 | 2.0% | 3 | 0.9% | |
| Yes/yes | 67 | 13.1% | 39 | 12.0% | |
| Mortality | 52 | 8.4% | 39 | 10.1% | 0.424 |
| Psychiatric diagnosis | |||||
| Depression | 305 | 49.4% | 237 | 61.6% | <0.001 |
| Bipolar disorder | 54 | 8.8% | 47 | 12.2% | 0.077 |
| Anxiety | 181 | 29.3% | 128 | 33.2% | 0.192 |
| Psychosis | 39 | 6.3% | 40 | 10.4% | 0.020 |
| PTSD | 22 | 3.6% | 14 | 3.6% | 0.953 |
| Adjustment disorder | 22 | 3.6% | 8 | 2.1% | 0.179 |
| Substance use disorders | |||||
| Any | 194 | 31.4% | 141 | 36.6% | 0.091 |
| Alcohol | 53 | 8.6% | 41 | 10.6% | 0.277 |
| Stimulants | 95 | 15.4% | 65 | 16.9% | 0.532 |
| Opioids | 15 | 2.4% | 10 | 2.6% | 0.870 |
| Sedatives | 1 | 0.2% | 1 | 0.3% | 0.736 |
| Cannabis | 7 | 1.1% | 9 | 2.3% | 0.139 |
| Other | 95 | 15.4% | 74 | 19.2% | 0.116 |
| Psychotropic medication | |||||
| Antidepressant | 256 | 41.5% | 169 | 43.9% | 0.454 |
| Antipsychotic | 67 | 10.9% | 56 | 14.5% | 0.084 |
| Sedative/hypnotic | 151 | 24.5% | 103 | 26.8% | 0.420 |
| Mood stabilizers | 37 | 6.0% | 26 | 6.8% | 0.631 |
| Antiretroviral medication | |||||
| Dolutegravir | 11 | 1.8% | 8 | 2.1% | 0.739 |
| Efavirenz | 134 | 21.7% | 66 | 17.1% | 0.078 |
| Rilpivirine | 2 | 0.3% | 0 | 0.0% | 0.263 |
| Raltegravir | 46 | 7.5% | 42 | 10.9% | 0.060 |
| Prior psychiatric office visit | 208 | 33.7% | 160 | 41.6% | 0.012 |
| Positive AUDIT-C | 168 | 27.8% | 122 | 32.2% | 0.143 |
| Missing | 13 | 2.1% | 6 | 1.6% | 0.703 |
| Lifetime drug use | |||||
| Any | 467 | 79.4% | 297 | 81.6% | 0.413 |
| Missing | 29 | 4.7% | 21 | 5.5% | 0.701 |
| Amphetamines | 325 | 54.3% | 225 | 60.3% | 0.064 |
| Missing | 18 | 2.9% | 12 | 3.1% | 1 |
| Opiates | 105 | 17.7% | 61 | 16.4% | 0.608 |
| Missing | 23 | 3.7% | 13 | 3.4% | 0.908 |
| Cocaine | 313 | 52.0% | 229 | 61.1% | 0.006 |
| Missing | 15 | 2.4% | 10 | 2.6% | 1 |
| Cannabis | 427 | 71.4% | 273 | 73.8% | 0.421 |
| Missing | 19 | 3.1% | 15 | 3.9% | 0.606 |
| Recent drug use | |||||
| Any | 255 | 43.7% | 175 | 48.2% | 0.172 |
| Missing | 33 | 5.3% | 22 | 5.7% | 0.917 |
| Amphetamines | 101 | 16.9% | 86 | 23.1% | 0.018 |
| Missing | 19 | 3.1% | 12 | 3.1% | 1 |
| Opiates | 16 | 2.7% | 11 | 3.0% | 0.81 |
| Missing | 25 | 4.1% | 14 | 3.6% | 0.871 |
| Cocaine | 34 | 5.7% | 34 | 9.1% | 0.038 |
| Missing | 16 | 2.6% | 13 | 3.4% | 0.599 |
| Cannabis | 204 | 34.2% | 134 | 36.3% | 0.497 |
| Missing | 20 | 3.2% | 16 | 4.2% | 0.561 |
| Mean | Sth. dev | Mean | Sth. dev | T-test | |
| Age | 48.0 | 11.2 | 48.6 | 10.0 | 0.378 |
| Time since HIV diagnosis (days) | 3814 | 3194 | 4189 | 3421 | 0.083 |
| Baseline PHQ-8 score | 14.1 | 3.7 | 16.9 | 4.4 | <0.001 |
| Median | IQR | Median | IQR | Wilcoxon | |
| Observations, all participants | 3 | 2,4 | 3 | 2,6 | <0.001 |
| Observations, remitters only | 3 | 2,4 | 3 | 2,5 | <0.001 |
Denominator for the Recent/Detectable variable is n = 512 patients without SI and n = 324 patients with SI, after excluding patients whose baseline viral load status is missing
SI, suicidal ideation; PI, Pacific Islander; HIV, human immunodeficiency virus; MSM, man who has sex with men; IDU, injection drug use; HAART, highly active antiretroviral therapy; PTSD, post-traumatic stress disorder; AUDIT-C, Alcohol Use Disorders Identification Test – Concise; PHQ-8, 8 non-SI items from the Patient Health Questionnaire-9; IQR, interquartile range
Unadjusted Analysis
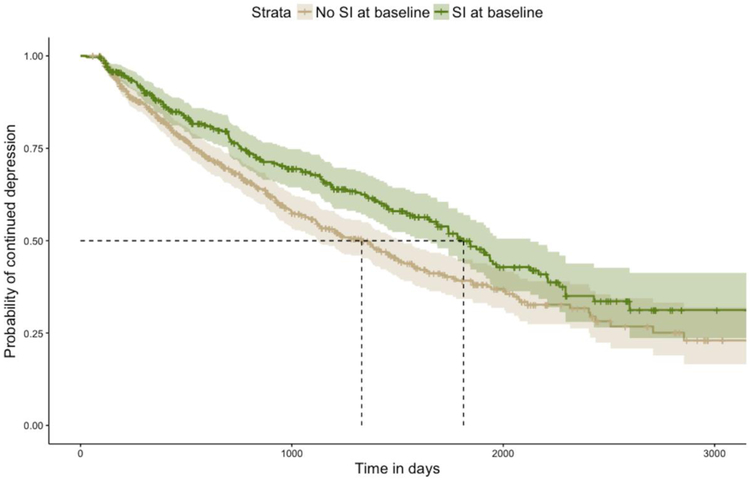
Kaplan-Meier curves for the distribution of the time to depression remission are presented in Figure 1, and results of time-to-remission analysis are presented in Table 2. Participants with SI at baseline had a significantly longer median time to depression remission than their counterparts without SI (1812 days versus 1330 days). Likewise participants with SI at baseline had lower 1-, 2-, and 5-year remission rates (0.120 versus 0.164, 0.236 versus 0.318, and 0.501 versus 0.608, respectively) and a significantly longer course of depressive illness (Gehan-Breslow-Wilcoxon p = 0.002). In Cox proportional hazard modeling, baseline SI was associated with an unadjusted hazard ratio (HR) for depression remission of 0.736, 95% CI = 0.604, 0.898 (p = 0.003), indicating a lower likelihood of attaining remission at any point in time. Concordance of this univariate Cox proportional hazard model was 0.542 (SE = 0.013).
Figure 1:
Kaplan-Meier curves for time to remission of depression
TABLE 2.
Analysis of time to depression remission, comparing patients with and without SI at baseline
| Cum.remitters at t | Non-remitters at t | Remission rate | Remission rate 95% CI |
|
|---|---|---|---|---|
| No SI at baseline | ||||
| 1 year | 93 | 432 | 0.164 | 0.133, 0.195 |
| 2 years | 165 | 289 | 0.318 | 0.276, 0.358 |
| 5 years | 257 | 74 | 0.608 | 0.551, 0.658 |
| SI at baseline | ||||
| 1 year | 43 | 288 | 0.120 | 0.086, 0.153 |
| 2 years | 78 | 214 | 0.236 | 0.188, 0.281 |
| 5 years | 135 | 71 | 0.501 | 0.431, 0.563 |
| Comparison of remission curves, Gehan-Breslow generalized Wilcoxon | p = 0.002 | |||
| Median time to remission, days | 95% CI | |||
| No SI at baseline | 1330 | 1126,1512 | ||
| SI at baseline | 1812 | 1644,2128 | ||
SI, suicidal ideation; CI, confidence interval
Covariate-Adjusted Analysis
Results of univariate Cox regression for covariates of interest are presented in Table 3. In addition to SI, significantly lower remission hazards were found for older age (HR = 0.986 per year, p = 0.003), psychiatric diagnoses of depression (HR = 0.711, p < 0.001) and anxiety (HR = 0.752, p = 0.011), active prescriptions for antidepressants (HR = 0.719, p = 0.001) and sedative/hypnotics (HR = 0.761, p = 0.017), lifetime use of any drug (HR = 0.774, p = 0.031), recent use of any drug (HR = 0.756, p = 0.006), a prior office visit with a psychiatrist (HR = 0.820, p = 0.049), and higher PHQ-8 score at baseline (HR = 0.943 per point, p < 0.001).
TABLE 3.
Results of univariate Cox regression analysis modeling hazard for depression remission by baseline characteristics of interest
| Variable | Coefficient | Hazard ratio | HR 95%CI | P.value |
|---|---|---|---|---|
| SI at baseline | −0.306 | 0.736 | 0.604, 0.898 | 0.003 |
| Sex | ||||
| Male | −0.018 | 0.983 | 0.748, 1.291 | 0.899 |
| Female | Reference | |||
| Race/ethnicity | ||||
| Non-Hispanic white | −0.278 | 0.757 | 0.448, 1.280 | 0.299 |
| Non-Hispanic black | −0.095 | 0.910 | 0.516, 1.604 | 0.744 |
| Hispanic | −0.016 | 0.984 | 0.578, 1.675 | 0.952 |
| Other | −0.216 | 0.806 | 0.370, 1.757 | 0.588 |
| Asian/PI | Reference | |||
| Age | −0.014 | 0.986 | 0.977, 0.995 | 0.003 |
| HIV risk factor | ||||
| MSM | 0.003 | 1.003 | 0.805, 1.249 | 0.978 |
| IDU | −0.271 | 0.762 | 0.563, 1.032 | 0.080 |
| Heterosexual | 0.031 | 1.031 | 0.808, 1.317 | 0.805 |
| Unknown/decline | 0.260 | 1.297 | 0.67, 2.512 | 0.440 |
| Time since HIV, days | <0.001 | 1.000 | 1.000, 1.000 | 0.333 |
| Era of HIV diagnosis | ||||
| Pre-HAART | Reference | |||
| Post-HAART | 0.099 | 1.104 | 0.906, 1.345 | 0.328 |
| Viral load* | ||||
| Undetectable | Reference | |||
| Detectable | 0.074 | 1.077 | 0.871, 1.332 | 0.495 |
| Recent (3 mo.) HIV diagnosis | 0.221 | 1.247 | 0.927, 1.678 | 0.144 |
| Recent/detectable | ||||
| No/no | Reference | |||
| No/yes | 0.026 | 1.026 | 0.807, 1.305 | 0.834 |
| Yes/no | 0.268 | 1.308 | 0.486, 3.519 | 0.595 |
| Yes/yes | 0.209 | 1.232 | 0.885, 1.716 | 0.217 |
| Psychiatric diagnoses | ||||
| Depression | −0.341 | 0.711 | 0.587, 0.861 | <0.001 |
| Bipolar disorder | −0.190 | 0.827 | 0.59, 1.159 | 0.270 |
| Anxiety | −0.285 | 0.752 | 0.604, 0.936 | 0.011 |
| Psychosis | −0.176 | 0.839 | 0.572, 1.23 | 0.368 |
| PTSD | −0.528 | 0.59 | 0.305, 1.142 | 0.118 |
| Adjustment disorder | −0.199 | 0.82 | 0.481, 1.398 | 0.465 |
| Substance use disorders | ||||
| Any | −0.068 | 0.935 | 0.765, 1.142 | 0.509 |
| Alcohol | 0.135 | 1.144 | 0.826, 1.585 | 0.418 |
| Stimulants | −0.106 | 0.899 | 0.696, 1.163 | 0.419 |
| Opioids | 0.137 | 1.147 | 0.646, 2.037 | 0.639 |
| Sedatives | 1.340 | 3.818 | 0.534, 27.31 | 0.182 |
| Cannabis | −0.488 | 0.614 | 0.229, 1.643 | 0.331 |
| Other | 0.002 | 1.002 | 0.775, 1.294 | 0.989 |
| Psychotropic medication | ||||
| Antidepressant | −0.330 | 0.719 | 0.592, 0.874 | 0.001 |
| Antipsychotic | −0.131 | 0.877 | 0.651, 1.181 | 0.387 |
| Sedative/hypnotic | −0.273 | 0.761 | 0.608, 0.952 | 0.017 |
| Mood stabilizers | 0.063 | 1.065 | 0.726, 1.563 | 0.746 |
| Antiretroviral medication | ||||
| Dolutegravir | 0.737 | 2.09 | 0.86, 5.082 | 0.104 |
| Efavirenz | −0.033 | 0.967 | 0.768, 1.218 | 0.778 |
| Rilpivirine | −0.190 | 0.827 | 0.116, 5.887 | 0.849 |
| Raltegravir | −0.132 | 0.877 | 0.631, 1.219 | 0.434 |
| Prior psychiatric office visit | −0.198 | 0.820 | 0.673, 0.999 | 0.049 |
| Positive AUDIT-C* | 0.098 | 1.102 | 0.894, 1.359 | 0.361 |
| Lifetime drug use* | ||||
| Any | −0.256 | 0.774 | 0.613, 0.977 | 0.031 |
| Amphetamines | −0.14 | 0.87 | 0.716, 1.055 | 0.157 |
| Opiates | −0.194 | 0.824 | 0.624, 1.087 | 0.171 |
| Cocaine | −0.081 | 0.922 | 0.761, 1.117 | 0.408 |
| Marijuana | −0.197 | 0.821 | 0.666, 1.013 | 0.066 |
| Recent drug use* | ||||
| Any | −0.280 | 0.756 | 0.619, 0.922 | 0.006 |
| Amphetamines | −0.165 | 0.848 | 0.649, 1.107 | 0.224 |
| Opiates | −0.114 | 0.892 | 0.461, 1.728 | 0.735 |
| Cocaine | 0.075 | 1.078 | 0.719, 1.615 | 0.717 |
| Marijuana | −0.188 | 0.829 | 0.673, 1.021 | 0.078 |
| Baseline PHQ-8 score | −0.058 | 0.943 | 0.92, 0.967 | <0.001 |
Observations deleted for missing data: N=165 for detectable viral load, N=19 for positive AUDIT-C, N=50 for any lifetime drug use, N=30 for lifetime amphetamine Use, N=36 for lifetime opiate Use, N=25 for lifetime cocaine use, N=34 for lifetime cannabis use, N=55 for any recent drug use, N=31 for recent amphetamine use, N=39 for recent opiate use, N=29 for recent cocaine use, N=36 for recent cannabis use SI, suicidal ideation; HR, hazard ratio; CI, confidence interval; PI, Pacific Islander; HIV, human immunodeficiency virus; MSM, man who has sex with men; IDU, injection drug use; HAART, highly active antiretroviral therapy; PTSD, post-traumatic stress disorder; AUDIT-C, Alcohol Use Disorders Identification Test – Concise; PHQ-8, 8 non-SI items from the Patient Health Questionnaire-9
Older age (HR = 0.985 per year, p = 0.003), depression diagnosis (HR = 0.801, p = 0.031), recent use of any drug (HR = 0.724, p = 0.002), and higher baseline PHQ-8 score (HR = 0.948 per point, p < 0.001) all predicted a longer course of depressive illness in the final multivariable Cox regression model (Table 4). We found no evidence for non-proportionality of hazard. Concordance of this multi-predictor Cox regression model was 0.594 (SE = 0.016). After controlling for the confounding effects of age and depression diagnosis and adjusting for depression severity as measured by PHQ-8, baseline SI was no longer a significant predictor of remission hazard, HR = 0.900, 95% CI=0.727, 1.115 (p = 0.334).
TABLE 4.
Final multivariable Cox regression model of hazard for depression remission by baseline SI and covariates of interest. N=55 observations excluded for missing drug use data
| Term | Coefficient | Hazard ratio | HR 95%CI | P value |
|---|---|---|---|---|
| SI at baseline | −0.105 | 0.900 | 0.727, 1.115 | 0.334 |
| Age | −0.015 | 0.985 | 0.975, 0.995 | 0.003 |
| Depression | −0.221 | 0.801 | 0.655, 981 | 0.031 |
| Baseline PHQ-8 score | −0.054 | 0.948 | 0.923, 0.973 | <0.001 |
| Any recent drug use | −0.323 | 0.724 | 0.592, 0.886 | 0.002 |
SI, suicidal ideation; HR, hazard ratio; PHQ-8, 8 non-SI items from the Patient Health Questionnaire-9
Without adjusting for PHQ-8 score, baseline SI was a significant predictor of prolonged time to remission in sensitivity analysis (see Table 5). The multivariable Cox regression model for baseline SI adjusted for age, antidepressant prescription at baseline, and recent use of any drug.
TABLE 5.
Sensitivity analysis: multivariable Cox regression model of hazard for depression remission by baseline SI and covariates of interest, baseline PHQ-8 score excluded
| Term | Coefficient | Hazard ratio | HR 95%CI | P.value |
|---|---|---|---|---|
| SI at baseline | −0.260 | 0.771 | 0.629, 0.945 | 0.012 |
| Age | −0.013 | 0.987 | 0.977, 0.997 | 0.011 |
| Antidepressant prescription | −0.264 | 0.768 | 0.625, 0.944 | 0.012 |
| Any recent drug use | −0.312 | 0.732 | 0.599, 0.895 | 0.002 |
N=55 observations excluded for missing drug use data
SI, suicidal ideation; HR, hazard ratio; PHQ-8, 8 non-SI items from the Patient Health Questionnaire-9
DISCUSSION:
This is the first longitudinal analysis of the association between SI and the chronicity of depressive symptoms in PLWH. In unadjusted analysis, we found that depressed PLWH presenting with SI in the outpatient setting may be expected to suffer depressive illness for a significantly longer period of time than their non-suicidal depressed peers. However, in multivariable and sensitivity analyses this finding was attributable to greater severity of depression rather than SI specifically. This association between depressive symptom severity and chronicity of illness has been observed among outpatients in general primary care, but it has never been shown in PLWH, a population with significant morbidity and mortality related to depression and suicide (Falola et al., 2017; Poutanen et al., 2007; Riihimaki et al., 2014; Stegenga et al., 2012). Our findings underscore the importance of routine screening for SI in PLWH as they may be at risk for greater chronicity of depression and poorer HIV and mental health outcomes. However, our findings also suggest that patients with greater severity of depressive symptoms, with or without SI, should be promptly identified and connected to care with similar urgency.
Particular attention is warranted to PLWH with a previous diagnosis of depression and those who are actively using drugs. The association between longer time to depression remission and baseline depression diagnosis may reflect a longer pre-study history of depressive illness, which has been observed to predict a more chronic depressive course (Holma et al., 2008). The finding that recent drug use predicted longer duration of depressive illness is consistent with findings from the general population that persons with substance use disorders and depression had a significantly lower likelihood of past year depression if in recovery (Agosti and Levin, 2010). In PLWH with depression, antidepressant treatment is recommended even in the setting of an active substance use disorder, as illicit drug use did not moderate antidepressant treatment response in a clinical trial of directly observed fluoxetine for depression (Grelotti et al., 2017). Our findings underscore the need for further study into the complex relationship between substance use and depression, as well as the importance of rigorous screening and treatment for substance use disorders in the outpatient context. This is especially relevant for PLWH, in whom substance use and depressive illness are highly comorbid (Bing et al., 2001) and considered psychosocial syndemics, i.e., “intertwined and mutually enhancing epidemics” (Singer and Clair, 2003) associated with poorer HIV treatment outcomes (Blashill et al., 2015; DeLorenze et al., 2010; Friedman et al., 2015).
While data from the United States general population suggest similar chronicity of depression between age groups (Kessler et al., 2010), older age predicted a longer course of depressive illness among PLWH in our analysis. And while no data is available on depression duration in this population, studies of middle-aged and older PLWH have shown a strikingly high prevalence of depressive symptoms and suicidal thoughts (Havlik, 2009; Kalichman et al., 2000). As a growing proportion of PLWH are over 50 years old (Mills et al., 2012), our findings reiterate the need for high-quality research characterizing the epidemiology, correlates, and course of depression in older adults with HIV.
LIMITATIONS:
This study has certain limitations. As we have conditioned our analysis on measurements taken at baseline, our ability to make causal inferences about determinants of depression chronicity is limited. Our model is structured with the assumption that all participants received comparable treatment for their affective symptoms. In practice, however, patients reporting SI on routine PROs are flagged for clinician review and may be more urgently referred to psychiatric treatment than their depressed peers without SI – potentially biasing the results toward the null. Mortality related to suicide, possibly more common among participants with SI, may have caused more loss to follow up among that subset of the sample. However, we found no significant difference in mortality between participants with and without SI at baseline. Patients were assessed at different times with variable frequency, according to their visit schedule and adherence to appointments. In our sample, more PHQ-9 observations were available for PLWH with SI at baseline rather than their non-suicidal counterparts (p < 0.001), which nevertheless reassures us that a delayed time to remission in this group is unlikely to be a function of delayed ascertainment. Rather than a clinical diagnostic instrument, we used a PHQ-8 score to define depression in our study participants and as such our analysis is subject to some degree of misclassification bias. However, the PHQ-9 depression screening tool is strongly predictive where prevalence of depression is high (Wittkampf et al., 2007). That being said, depressive symptoms are not specific to major depressive disorder, and methods to identify depressive disorders using the electronic medical record are imperfect (DiPrete et al., 2018). If depressive symptoms stem from significant health issues and are not due to a primary mood disorder such as major depressive disorder, this may contribute to depression chronicity as we have defined it. Regardless, clinicians should work to identify and address the underlying cause of these serious and often debilitating symptoms.
CONCLUSION:
Our analysis provides insight into the clinical course of depression and SI in a clinical sample of PLWH engaged in primary care. Self-reported SI was associated with a significantly longer time to remission in unadjusted analysis, but not when accounting for baseline depression severity. Severe depression as measured by higher PHQ-8 scores, older age, depression diagnosis, and recent drug use all predicted greater chronicity of depression. As our study is an associational one, further exploration of SI, depression severity, and remission with methods suited to infer causality is needed. Nevertheless, our study is part of a larger body of literature which underscores the importance of depression screening and urgently connecting PLWH to appropriate psychiatric care in the event of SI or severe depressive symptoms.
Supplementary Material
HIGHLIGHTS:
People living with HIV (PLWH) have higher rates of depression and suicidal ideation
Chronicity of depression worsens HIV treatment outcomes
Alone, suicidal ideation predicts depression chronicity in PLWH
Together, age, recent drug use, and severe depression predict depression chronicity
Those with HIV and severe depression, suicidal or not, should be engaged in care
ACKNOWLEDGEMENTS:
The authors are grateful to Huifang Qin of the San Diego Center for AIDS Research for the curation and provision of de-identified clinical data.
ROLE OF THE FUNDING SOURCE:
This project was partially supported by the National Institutes of Health through a grant to the UC San Diego Center for AIDS Research (NIAID 5 P30 AI036214) and 1TL1TR001443 of CTSA funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- HIV
human immunodeficiency virus
- PLWH
people living with HIV
- SI
suicidal ideation
- HAART
highly active antiretroviral therapy
- AIDS
acquired immunodeficiency syndrome
- PRO
patient-reported outcomes
- PHQ
patient health questionnaire
- MSM
man who has sex with men
- IDU
injection drug use
- ICD
International Classification of Diseases
- PTSD
post-traumatic stress disorder
- ASSIST
Alcohol, Smoking and Substance Involvement Screening Test
- AUDIT-C
Alcohol Use Disorders Identification Test — Concise
- ARV
antiretroviral
- HR
hazard ratio
- SE
standard error
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
INSTITUTIONAL BOARD REVIEW:
The Institutional Review Board of UC San Diego exempted this study from review.
CONFLICT OF INTEREST:
Dr. Grelotti served as a paid consultant for Greenwich Biosciences for contributions to the Cannabis Educators Working Group. The authors have no other competing financial interests to report.
REFERENCES:
- Agosti V, Levin FR, 2010. The Effects of Alcohol and Drug Dependence on the Course of Depression. Am. J. Addict 15, 71–75. [DOI] [PubMed] [Google Scholar]
- Badiee J, Moore DJ, Atkinson JH, Vaida F, Gerard M, Duarte NA, Franklin D, Gouaux B, McCutchan JA, Heaton RK, McArthur J, Morgello S, Simpson D, Collier A, Marra CM, Gelman B, Clifford D, Grant I, 2012. Lifetime suicidal ideation and attempt are common among HIV+ individuals. J. Affect. Disord 136, 993–9. https://doi.Org/10.1016/j.jad.2011.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M, 2001. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry 58, 721–8. [DOI] [PubMed] [Google Scholar]
- Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, Mimiaga MJ, Safren SA, 2015. Psychosocial Syndemics are Additively Associated with Worse ART Adherence in HIV-Infected Individuals. AIDS Behav. 19, 981–6. 10.1007/s10461-014-0925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR, 2007. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol. Clin. Exp. Res 31, 1208–17. https://doi.Org/10.1111/j.1530-0277.2007.00403.x [DOI] [PubMed] [Google Scholar]
- Carrieri M, Marcellin F, Fressard L, Préau M, Sagaon-Teyssier L, Suzan-Monti M, Guagliardo V, Mora M, Roux P, Dray-Spira R, Spire B, Group A-VS, 2017. Suicide risk in a representative sample of people receiving HIV care: Time to target most-at-risk populations (ANRS VESPA2 French national survey). PLoS One 12, e0171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalan J, Harding R, Sibley E, Clucas C, Croome N, Sherr L, 2011. HIV infection and mental health: suicidal behaviour--systematic review. Psychol. Health Med 16, 588–611. https://doi.Org/10.1080/13548506.2011.582125 [DOI] [PubMed] [Google Scholar]
- Cholera R, Pence BW, Bengtson AM, Crane HM, Christopoulos K, Cole SR, Fredericksen R, Gaynes BN, Heine A, Mathews WC, Mimiaga MJ, Moore R, Napravnik S, O’Clerigh C, Safren S, Mugavero MJ, 2017. Mind the Gap: Gaps in Antidepressant Treatment, Treatment Adjustments, and Outcomes among Patients in Routine HIV Care in a Multisite U.S. Clinical Cohort. PLoS One 12, e0166435 10.1371/journal.pone.0166435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE, 2001. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am. J. Psychiatry 158, 725–730. https://doi.Org/10.1176/appi.ajp.158.5.725 [DOI] [PubMed] [Google Scholar]
- DeLorenze G, Satre D, Quesenberry C, Tsai A, Weisner C, 2010. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients. AIDS Patient Care STDs 24, 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPrete BL, Pence BW, Grelotti DJ, Gaynes BN, 2018. Measure of depression treatment among patients receiving HIV primary care: Whither the truth? J. Affect. Disord 230, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshun-Wilson I, Siegfried N, Akena D, Stein D, Obuku E, Joska J, 2018. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev 1:CD008525. 10.1002/14651858.CD008525.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falola M, Limdi N, Shelton R, 2017. Clinical and Genetic Predictors of Delayed Remission After Multiple Levels of Antidepressant Treatment: Toward Early Identification of Depressed Individuals for Advanced Care Options. J. Clin. Psychiatry 78, e1291. [DOI] [PubMed] [Google Scholar]
- Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, Hsu R, Fusco J, Quercia R, Aboud M, Curtis L, 2017. Psychiatric Symptoms in Patients Receiving Dolutegravir. J. Acquir. Immune Defic. Syndr 74, 423–431. 10.1097/QAI.0000000000001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D, Levenson J, 2015. Rilpivirine and Depression. Psychosomatics 56, 711–2. https://doi.Org/10.1016/j.psym.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Friedman MR, Stall R, Silvestre AJ, Wei C, Shoptaw S, Herrick A, Surkan PJ, Teplin L, Plankey MW, 2015. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS 29, 1087–96. 10.1097/QAD.0000000000000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Hammer GP, Dilley JW, Karasic DH, Sorensen JL, Bangsberg DR, Tsai AC, 2017. Does substance use compromise depression treatment in persons with HIV? Findings from a randomized controlled trial. AIDS Care 29, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlik R, 2009. Health status, comorbidities, and health-related quality of life, in: Brennan M, Karpiak S, Cantor M, Shippy R (Eds.), Research on Older Adults with HIV: An in-Depth Examination of an Emerging Population. Nova Science, New York, pp. 13–25. [Google Scholar]
- Hoffmann C, Welz T, Sabranski M, Kolk M, Wolf E, Stellbrink H-J, Wyen C, 2017. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 18, 56–63. 10.1111/hiv.12468 [DOI] [PubMed] [Google Scholar]
- Holma K, Holma I, Melartin T, Rytsälä H, Isometsä E, 2008. Long-term outcome of major depressive disorder in psychiatric patients is variable. J. Clin. Psychiatry 69, 196. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, De Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S, 2008. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 103, 1039–1047. https://doi.Org/10.1111/j.1360-0443.2007.02114.x [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J, for the HIV Epidemiology Research Study Group, 2001. Mortality, CD4 Cell Count Decline, and Depressive Symptoms Among HIV-Seropositive Women. JAMA 285, 1466 10.1001/jama.285.11.1466 [DOI] [PubMed] [Google Scholar]
- Jia CX, Mehlum L, Qin P, 2012. AIDS/HIV infection, comorbid psychiatric illness, and risk for subsequent suicide: A nationwide register linkage study. J. Clin. Psychiatry 73, 1315–1321. 10.4088/JCP.12m07814 [DOI] [PubMed] [Google Scholar]
- Kalichman S, Heckman T, Kochman A, Sikkema K, Bergholte J, 2000. Depression and thoughts of suicide among middle-aged and older persons living with HIV-AIDS. Psychiatr. Serv. 51, 903–7. [DOI] [PubMed] [Google Scholar]
- Kang CR, Bang JH, Cho S-I, Kim KN, Lee H-J, Ryu BY, Cho SK, Lee YH, Oh M-D, Lee J-K, 2016. Suicidal ideation and suicide attempts among human immunodeficiency virus-infected adults: differences in risk factors and their implications. AIDS Care 28, 306–13. 10.1080/09540121.2015.1093593 [DOI] [PubMed] [Google Scholar]
- Keiser O, Spoerri A, Brinkhof MWG, Hasse B, Gayet-Ageron A, Tissot F, Christen A, Battegay M, Schmid P, Bernasconi E, Egger M, 2010. Suicide in HIV-infected individuals and the general population in Switzerland, 1988-2008. Am. J. Psychiatry 167, 143–150 10.1176/appi.ajp.2009.09050651. [DOI] [PubMed] [Google Scholar]
- Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, Dunne M, Burrows G, 1998. Suicidal ideation, suicide attempts, and HIV infection. Psychosomatics 39, 405–15. 10.1016/S0033-3182(98)71299-X [DOI] [PubMed] [Google Scholar]
- Kessler R, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V, 2010. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol. Med 40, 225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheloufi F, Allemand J, Mokhtari S, Default A, 2015. Psychiatric disorders after starting dolutegravir: report of four cases. AIDS 29, 1723–5. 10.1097/QAD.0000000000000789 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer R, Williams J, 2001. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med 16, 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, Löwe B, 2010. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen. Hosp. Psychiatry 32, 345–359. https://doi.Org/10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine T, Spitzer R, Williams J, Berry J, Mokdad A, 2009. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord 114, 163–173. [DOI] [PubMed] [Google Scholar]
- López JD, Shacham E, Brown T, 2018. Suicidal Ideation Persists Among Individuals Engaged in HIV Care in the Era of Antiretroviral Therapy. AIDS Behav. 22, 800–805. 10.1007/s10461-016-1666-5 [DOI] [PubMed] [Google Scholar]
- Mills E, Baringhausen T, Negin J, 2012. HIV and Aging — Preparing for the Challenges Ahead. N. Engl. J. Med 366, 1270–1273. [DOI] [PubMed] [Google Scholar]
- Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE, Gulick RM, Na L, O’Keefe L, Robertson KR, Tierney C, 2014. Association between Efavirenz as Initial Therapy for HIV-1 Infection and Increased Risk of Suicidal Ideation, Attempted, or Completed Suicide. Ann. Intern. Med 161, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L, 2015. Depression in HIV infected patients: a review. Curr. Psychiatry Rep 17, 530 10.1007/s11920-014-0530-4 [DOI] [PubMed] [Google Scholar]
- Peltzer K, Szrek H, Ramlagan S, Leite R, Chao L-W, 2015. Depression and social functioning among HIV-infected and uninfected persons in South Africa. AIDS Care 27, 41–6. 10.1080/09540121.2014.946383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence B, Gaynes B, Adams J, Thielman N, Heine A, Mugavero M, McGuinness T, Raper J, Willig J, Shirey K, Ogle M, Turner E, Quinlivan E, 2015. The effect of antidepressant treatment on HIV and depression outcomes: results from a randomized trial. AIDS 29, 1975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, Moore RD, Grelotti DJ, O’Cleirigh C, Mugavero MJ, 2018. Association of Increased Chronicity of Depression With HIV Appointment Attendance, Treatment Failure, and Mortality Among HIV-Infected Adults in the United States. JAMA psychiatry, 10.1001/jamapsychiatry.2017.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, O’Donnell JK, Gaynes BN, 2012. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 26, 656–8. https://doi.Org/10.1097/QAD.0b013e3283519aae [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S, Jacobserb L, Fishman B, 1990. Suicidal Ideation and HIV Testing. JAMA 263, 679–682. [PubMed] [Google Scholar]
- Poutanen O, Mattila A, Seppala N, Groth L, Koivisto A, Salokangas R, 2007. Seven-year outcome of depression in primary and psychiatric outpatient care: results of the TADEP (Tampere Depression) II Study. Nord. J. Psychiatry 61,62–70. [DOI] [PubMed] [Google Scholar]
- Protopopescu C, Raffi F, Brunet-Francois C, Salmon D, Verdon R, Reboud P, Carrieri M, Leport C, Spire B, Michel L, APROCO-COPILOTE (ANRS CO8) Study Group, 2012. Incidence, medical and socio-behavioral predictors of psychiatric events in an 11-year follow-up of HIV-infected patients on antiretroviral therapy. Antivir. Ther 17, 1079–83. [DOI] [PubMed] [Google Scholar]
- Riihimaki K, Vuorilehto M, Isometsa E, 2014. Five-year outcome of major depressive disorder in primary health care. Psychol. Med 44, 1369–79. [DOI] [PubMed] [Google Scholar]
- Simon GE, Rutter CM, Peterson D, Oliver M, Whiteside U, Operskalski B, Ludman EJ, 2013. Does Response on the PHQ-9 Depression Questionnaire Predict Subsequent Suicide Attempt or Suicide Death? Psychiatr. Serv 64, 1195–1202. 10.1176/appi.ps.201200587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, DiMatteo MR, 2014. Depression treatment enhances adherence to antiretroviral therapy: a metaanalysis. Ann. Behav. Med 47, 259–69. 10.1007/s12160-013-9559-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Clair S, 2003. Syndemics and Public Health: Reconceptualizing Disease in Bio-Social Context. Med. Anthropol. Q 17, 423–41. https://doi.Org/10.1525/maq.2003.17.4.423 [DOI] [PubMed] [Google Scholar]
- Stegenga B, Kamphuis K, King M, Nazareth I, Geerlings M, 2012. The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc. Psychiatr. Psychiatr. Epidemiol 47, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC, 2007. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen. Hosp. Psychiatry 29, 388–395. https://doi.Org/10.1016/j.genhosppsych.2007.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.