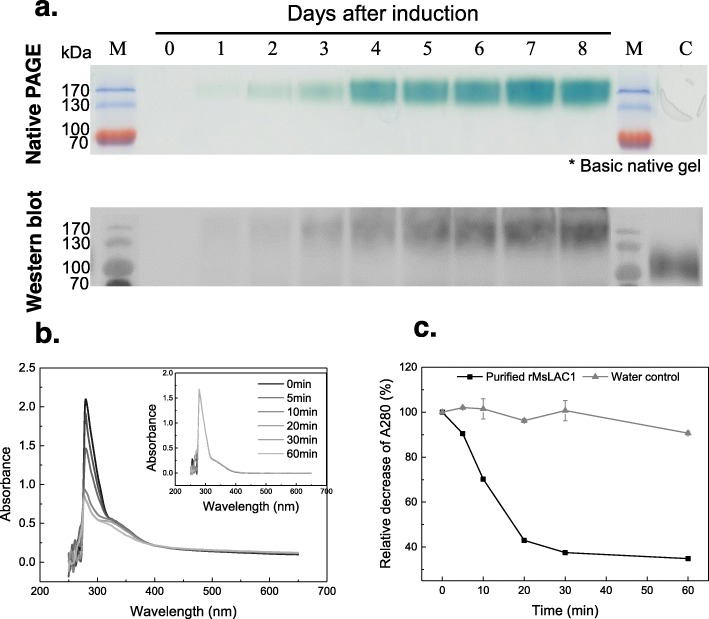
Fig. 6.
Expression of recombinant MsLAC1 protein and oxidation of sinapyl alcohol by purified recombinant protein. a The protein was expressed in P. pastoris and analyzed by Native PAGE and Western blot. After methanol induction samples of culture medium were collected at daily intervals, cleared by centrifugation, and 10 μl of supernatant were filtered and loaded. The zymogram was stained with 10 mM ABTS. Western blot was developed with anti-MYC antibody. M: PageRular Prestained Protein Ladder, 10–170 kDa. C: Crude extract from fermentation broth of P. pastoris transformed with pPICZαAfeh, 8 days after induction. Sinapyl alcohol (final concentration 1 mM) was incubated with 0.002 U recombinant MsLAC1 protein in 50 mM acetate buffer, pH 3.0. b Spectra of reaction mixtures (250 nm – 650 nm) were recorded at indicated time intervals (the insert indicates the control with substrate only). c Time course of absorption change at 280 nm. The initial absorbance at 280 nm was set as 100% individually for the calculation of absorbance decrease with purified protein and water control