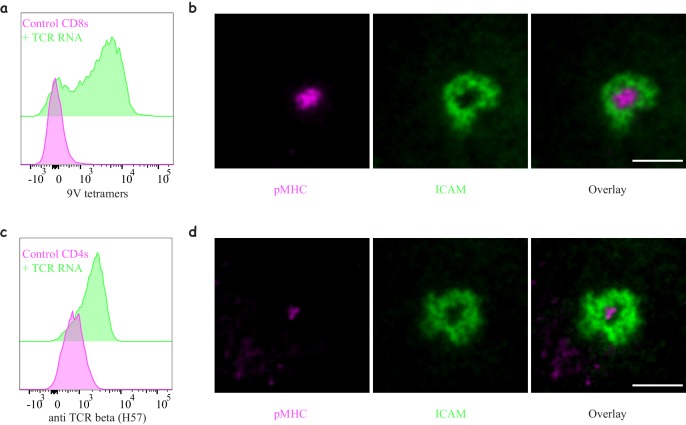
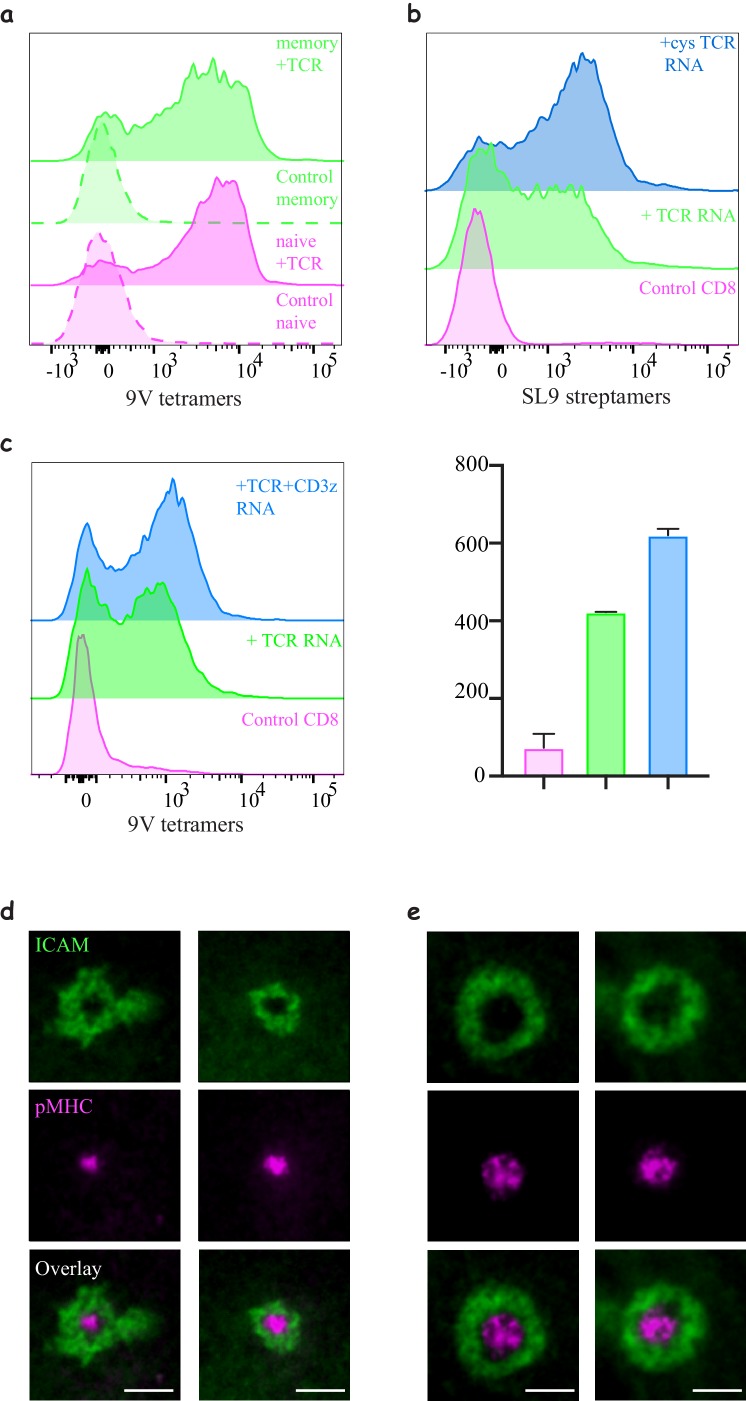
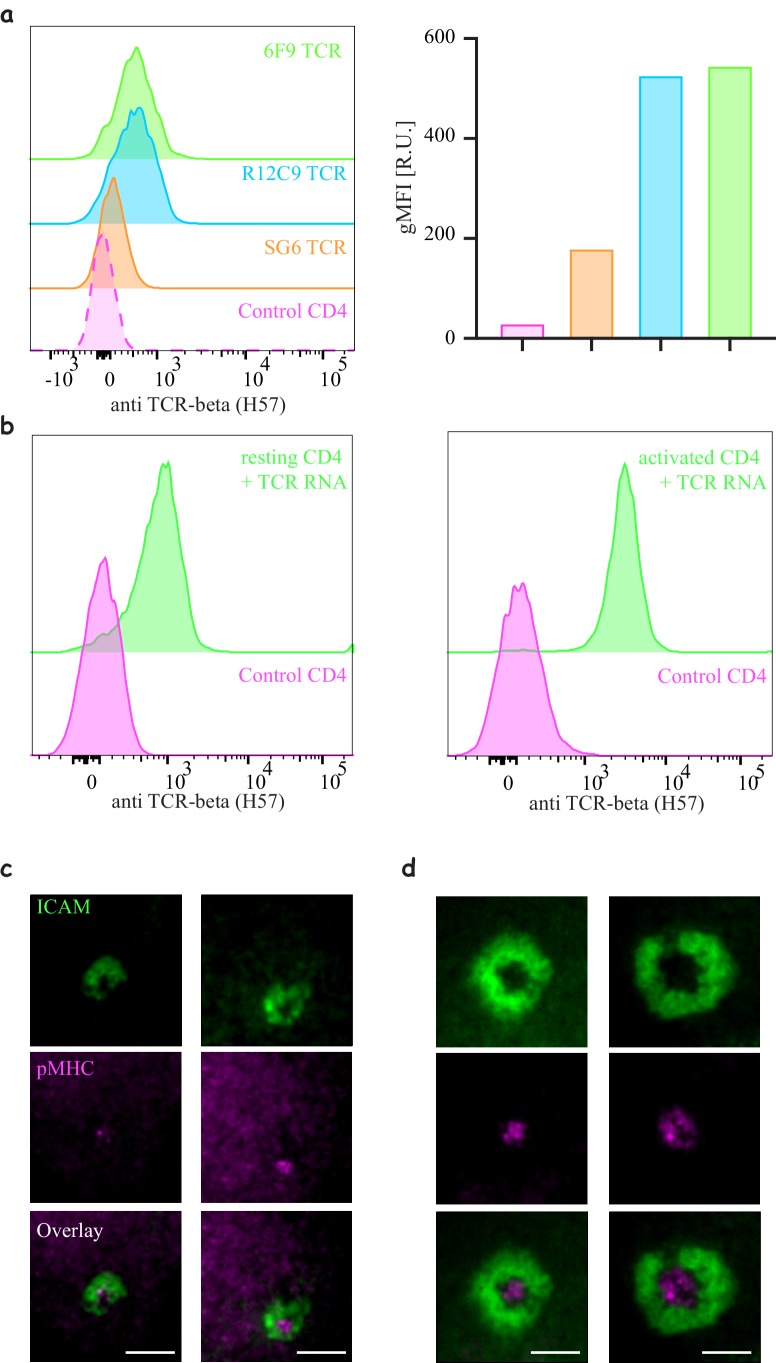
Figure 2. Engineering antigen-specific T-cells.
(a) Expression of 1G4 TCR in naïve CD8 T-cells upon mRNA electroporation detected using NY-ESO-9V/HLA-A2 tetramer,~80% positive (Representative of N = 13). (b) Formation of an immunological synapse by 1G4-expressing naïve CD8 T-cells on supported lipid bilayers (SLBs) with cSMAC enriched with NY-ESO-9V/A2 pMHC (magenta) surrounded by an LFA1/ICAM1 ring (green). Representative of >3 independent repeats. (c) Expression of 6F9 TCR in naïve CD4 T-cells detected using an antibody against the constant region of mouse TCRβ,~67% positive (Representative of N = 15). (d) Formation of an immunological synapse by 6F9-expressing naïve CD4 T-cells on SLB containing MAGE/DP4 pMHC (magenta). Representative of >3 independent repeats. Scale bars = 5 μm.