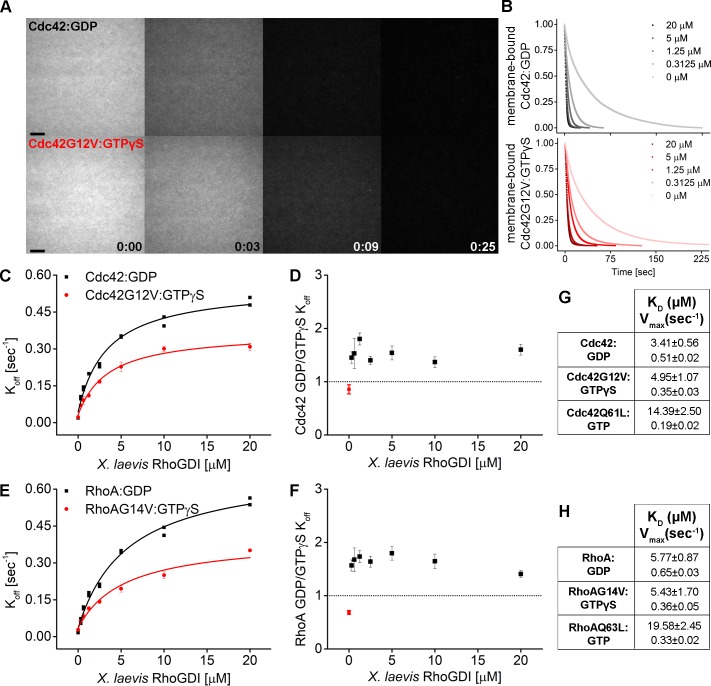
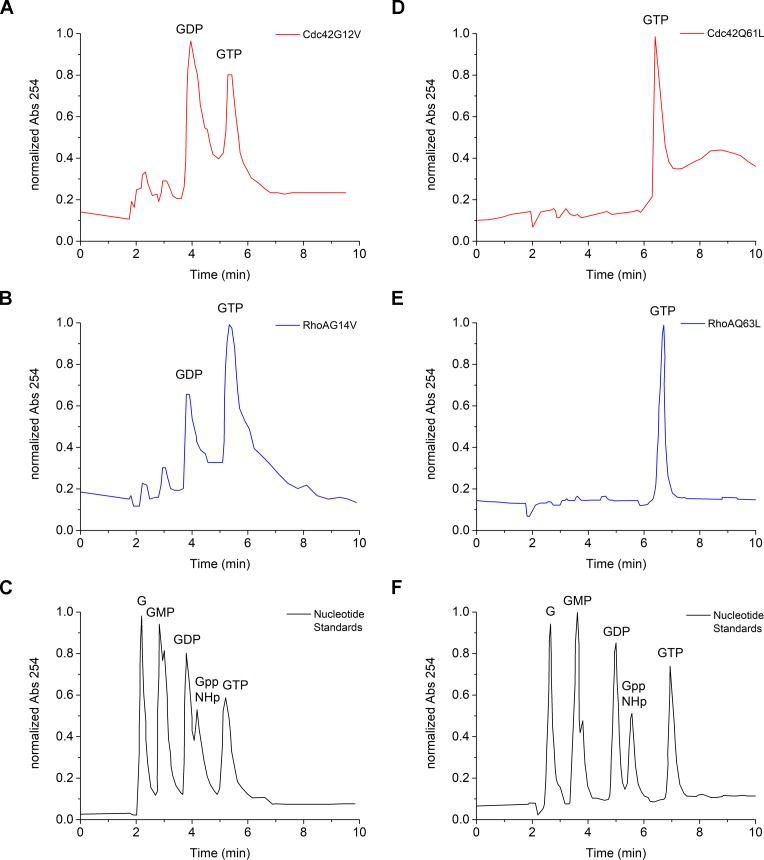
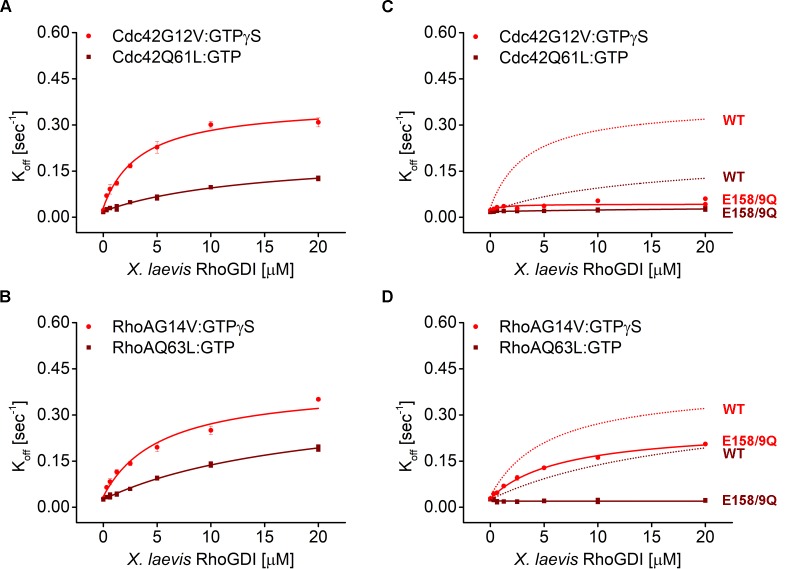
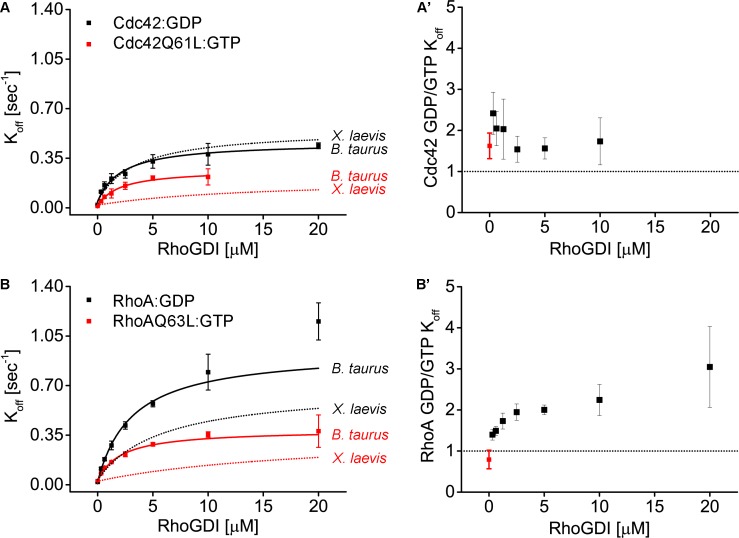
Figure 7. RhoGDI extracts both inactive and active RhoGTPases from membranes in vitro.
(A) Wash off experiments: prenylated Cdc42 in both inactive (Cdc42:GDP) and constitutively-active (Cdc42G12V:GTPγS) states were reconstituted on SLBs and washed in presence of 5 μM GDI. Time lapse images at selected time points are shown; (B) Quantification of wash off experiments in which the concentration of GDI was titrated between 0 and 20 μM; (C) Koff values obtained for inactive and constitutively-active Cdc42G12V fitting the decay curves with a monoexponential decay function are plotted against GDI concentration. Extraction rates were fitted with a hyperbolic function; fitting parameters Kd and Vmax are reported in table G; (D) Ratio of Koff obtained for inactive and constitutively-active Cdc42G12V at the same GDI concentration; (E–F, H) Same as in C-D and G for inactive (Rho:GDP) and constitutively-active (RhoG14V:GTPγS) Rho. Scale bar 10 μm.