Abstract
Background
Regenerating islet-derived protein 3α (REG3α) is an antimicrobial peptide secreted by intestinal Paneth cells. Circulating REG3α has been identified as a gut damage marker in inflammatory bowel diseases. People living with human immunodeficiency virus (PWH) on antiretroviral therapy (ART) present with an abnormal intestinal landscape leading to microbial translocation, persistent inflammation, and development of non-AIDS comorbidities. Herein, we assessed REG3α as a marker of gut damage in PWH.
Methods
Plasma from 169 adult PWH, including 30 elite controllers (ECs), and 30 human immunodeficiency virus (HIV)–uninfected controls were assessed. REG3α plasma levels were compared with HIV disease progression, epithelial gut damage, microbial translocation, and immune activation markers.
Results
Cross-sectionally, REG3α levels were elevated in untreated and ART-treated PWH compared with controls. ECs also had elevated REG3α levels compared to controls. Longitudinally, REG3α levels increased in PWH without ART and decreased in those who initiated ART. REG3α levels were inversely associated with CD4 T-cell count and CD4:CD8 ratio, while positively correlated with HIV viral load in untreated participants, and with fungal product translocation and inflammatory markers in all PWH.
Conclusions
Plasma REG3α levels were elevated in PWH, including ECs. The gut inflammatory marker REG3α may be used to evaluate therapeutic interventions and predict non-AIDS comorbidity risks in PWH.
Keywords: HIV, gut damage, REG3α, microbial translocation, inflammation
Human immunodeficiency virus (HIV) infection is characterized by a rapid decline in mucosal CD4 T-cell count, early epithelial gut damage, and subsequent translocation of microbial products into the systemic circulation [1]. Epithelial gut damage and microbial translocation have been linked with inflammation, HIV disease progression, and occurrence of non-AIDS comorbidities such as cardiovascular and fatty liver diseases, neurocognitive dysfunctions, and cancer in people living with HIV (PWH) under antiretroviral therapy (ART) [2, 3].
In the absence of ART, the majority of PWH, called progressors, have an abnormal gastrointestinal landscape characterized by villous atrophy, crypt hyperplasia, loosened tight junctions, gastrointestinal inflammation, and increased intestinal permeability [4–7]. Despite long-term ART, damage to the gut mucosa persists in PWH [8–10]. However, elite controllers (ECs), a rare subset of PWH who maintain undetectable viral load (VL) without ART, present with lower levels of gut mucosal damage compared with progressors [11].
The mechanism behind persistent gut damage in PWH is not fully understood [12]. Epithelial gut damage has been observed to appear prior to immune changes in simian immunodeficiency virus (SIV)–infected rhesus macaques [7]. Deterioration of the gastrointestinal landscape in PWH and SIV-infected rhesus macaques has been shown to cause translocation of bacterial and fungal products contributing to chronic immune activation and the development of non-AIDS comorbidities [1, 12–15]. Thus, understanding the underlying mechanisms of gut damage in PWH may help to develop novel therapeutic strategies to reduce systemic immune activation and subsequent development of non-AIDS comorbidities in PWH on ART.
Markers of gut barrier integrity are commonly used in clinical research, as assessing the entire gut epithelium by endoscopy remains difficult [16]. Blood markers of microbial translocation such as lipopolysaccharide (LPS) and soluble CD14 (sCD14) are used as indirect measures of gut epithelium integrity. Circulating intestinal fatty acid binding protein (I-FABP), an intracellular protein constitutively expressed in enterocytes, is commonly used. Upon intestinal cell death, I-FABP is released into the mucosa and subsequently translocates into the blood in inflammatory bowel diseases (IBDs) [17, 18]. In PWH, circulating levels of I-FABP were found to be elevated in HIV progressors but not in ECs [11, 19]. However, some studies found an increase in I-FABP levels after ART initiation, which does not mirror the decrease of microbial translocation and inflammation observed after ART initiation [19–21]. Moreover, we and others have shown that circulating I-FABP levels are not associated with some markers of microbial translocation and inflammation in ART-treated PWH [15, 22].
Immunoglobulin A (IgA), antimicrobial peptides, the mucus layer, and tight junctions establish a barrier preventing translocation of commensal bacteria and pathogens into systemic circulation. Regenerating islet-derived protein 3α (REG3α), also called HIP (hepatocarcinoma-intestine-pancreas) or PAP (pancreatitis-associated protein), is a C-type lectin antimicrobial peptide constitutively secreted in the gut lumen by Paneth cells [23]. REG3α is selectively produced in the small intestine upon bacterial colonization as its homolog REG3γ is absent in germ-free mice [24]. Upon intestinal stress, REG3α production is increased to help contain bacterial infection by binding to peptidoglycan and killing gram-positive bacteria [23, 24]. Upon loss of gut barrier integrity, REG3α can cross the epithelium, translocate into the lamina propria, and enter the systemic circulation [25]. Hence, circulating levels of REG3α are considered to be a marker of gut damage during enteropathies such as Crohn and celiac diseases, ulcerative colitis, and graft-vs-host disease (GVHD) [25–27].
Herein, we investigated whether the gut damage marker REG3α was elevated in the plasma of PWH. We compared REG3α levels in PWH in early and chronic phases of HIV infection, in participants who initiated ART or remained untreated, as well as in ECs. We also assessed the association between plasma levels of REG3α and markers of HIV disease progression, microbial translocation, and inflammation.
METHODS
Study Design
A total of 169 adult PWH were enrolled from the Montreal Primary HIV Infection Study, from patients followed at the Chronic Viral Illness Service (CVIS) at the McGill University Health Centre (MUHC), from the Canadian HIV and Aging Cohort (CHACS) [28] and from the Canadian cohort of HIV-Infected Slow Progressors. PWH were categorized into those in early HIV infection (n = 51), defined as being within 6 months of the estimated date of HIV acquisition determined using the Department of Health and Human Services–National Institutes of Health Acute HIV Infection and Early Diagnosis Research Program guidelines [29, 30], or those in chronic HIV infection who were either untreated (n = 22) or ART-treated (n = 66). Samples from 30 ECs maintaining plasma viremia <1.7 log10 copies/mL and CD4 T-cell count >200 cells/µL in the absence of ART were analyzed. Groups of PWH were compared to 30 HIV-uninfected controls who were mostly partners of PWH, recruited from the CVIS at the MUHC and the CHACS (Supplementary Figure 1). We prospectively followed 22 PWH for 2 years. Ten participants were followed from the early phase of the infection before and after at least 1 year on ART, while 12 ART-naive persons with early HIV infection were followed and remained without ART (Table 1, Supplementary Figure 1). All participants were fasting at the time of blood collection. Participants did not present with any acute condition or history of IBD. To account for potential confounders, we recorded renal/pancreatic/liver functions, serum lipid levels, viral coinfections, and the usage of antibiotics. Blood samples were collected to perform clinical measurements. Plasma and peripheral blood mononuclear cells were isolated and stored at –80°C and in liquid nitrogen, respectively, until used.
Table 1.
Participant Characteristics
| Early HIV Infection | Chronic HIV Infection | ECs | Controls | ||
|---|---|---|---|---|---|
| (n = 88) | |||||
| ART Naive | ART Treated | ||||
| Characteristic | (n = 51) | (n = 22) | (n = 66) | (n = 30) | (n = 30) |
| Age, y | |||||
| Median | 34 | 38 | 55 | 43 | 58 |
| IQR | 28–44 | 33–50 | 47–61 | 36–50 | 52–61 |
| Sex, % | |||||
| Women | 3 | 23 | 11 | 27 | 27 |
| Men | 97 | 77 | 89 | 73 | 73 |
| CD4 count, cells/µL | |||||
| Median | 460 | 220 | 595 | 576 | 821 |
| IQR | 310–640 | 35–345 | 416–700 | 498–745 | 519–1022 |
| Range | 210–1680 | 3–489 | 54–1251 | 290–1090 | 281–1173 |
| CD8 count, cells/µL | |||||
| Median | 810 | 770 | 720 | 660 | 373 |
| IQR | 620–1040 | 407–1147 | 552–968 | 433–955 | 273–536 |
| Range | 279–2590 | 54–1425 | 140–1475 | 260–1560 | 188–843 |
| CD4:CD8 ratio | |||||
| Median | 0.57 | 0.19 | 0.77 | 0.86 | 2.08 |
| IQR | 0.39–0.8 | 0.06–0.43 | 0.54–1.12 | 0.69–1.24 | 1.22–3.01 |
| Viral load, log10 copies/mL | |||||
| Median | 4.5 | 5.1 | <1.7 | <1.7 | NA |
| IQR | 3.8–5.0 | 4.4–5.5 | 1.7-1.7 | NA | |
| Range | 1.3–7.5 | 3.9–5.9 | 1.6–1.7 | NA | |
| Time to initiation of ART, y | |||||
| Median | NA | NA | 3.0 | NA | NA |
| IQR | 0.6–7.0 | ||||
| Range | 0–23.8 | ||||
| ART duration, y | |||||
| Median | NA | NA | 13.6 | NA | NA |
| IQR | 1.6–17.8 | ||||
| Range | 0.2–25.4 | ||||
Abbreviations: ART, antiretroviral therapy; ECs, elite controllers; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
Clinical Laboratory Measurements
Plasma HIV type 1 (HIV-1) p24 antigen/antibody and a confirmatory Western blot test diagnosed HIV infection as previously reported [15]. Quantification of plasma VL was done using the Abbott RealTime HIV-1 assay (Abbott Laboratories). Total immunoglobulin G (IgG), immunoglobulin M (IgM), and IgA levels were measured in serum using an Olympus AU58000 (Beckman Coulter). CD4 and CD8 T-cell counts were measured using 4-color flow cytometry.
Markers of Epithelial Gut Damage, Microbial Translocation, Inflammation, and Global B-Cell Activation
REG3α and I-FABP were quantified in plasma using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems and Hycult Biotech, respectively). Anti-cytomegalovirus (CMV) IgG concentrations were measured using the anti-CMV IgG enzyme immunoassay test kit (GenWay Biotech). LPS was quantified using a human LPS ELISA kit (Cusabio). sCD14 was quantified by immunoassay (Quantikine, R&D Systems). Plasma (1→3)-β-d-glucan level (β-d-glucan) was measured by the Fungitell Limulus Amebocyte Lysate assay (Associates of Cape Cod). Plasma levels of interleukin 6 (IL-6), interleukin 8 (IL-8), and tumor necrosis factor (TNF)–α were measured by MSD multiplexes (MesoScale Discovery) [31]. Interleukin 22 (IL-22) levels were quantified by ELISA (R&D Systems). Kynurenine and tryptophan plasma levels were measured using an automated online solid-phase extraction liquid chromatographic tandem mass spectrometric method [10]. The kynurenine-to-tryptophan ratio was calculated as a measure of indoleamine-2,3-dioxygenase (IDO-1) enzyme activity. All measurements were done in duplicate as previously reported [31]. Percentage of activated CD4 and CD8 T-cells was determined by flow cytometry analysis of the coexpression of HLA-DR and CD38 [15].
Statistical Analyses
Statistical analyses were conducted using GraphPad Prism 6.0 software. Comparisons were conducted using nonparametric Mann–Whitney U test and Kruskal–Wallis test with Dunn correction for multiple variables. Correlations were performed using a nonparametric Spearman test. An α level of 5% was used for statistical significance. Multivariate analysis was performed using IBM SPSS 24.0 software.
Ethical Considerations
All study participants provided written consent for enrollment and ethical approval was obtained from the MUHC and the Centre Hospitalier de l’Université de Montréal research ethics boards. The study was conducted in accordance with the Declaration of Helsinki.
RESULTS
Study Participant Characteristics
Participants had a median age of 48 (interquartile range [IQR], 36–56) years, and 87.1% were male. Untreated PWH had a lower CD4 T-cell count with a median of 480 (IQR, 321–658) cells/µL, whereas CD4 T-cell count was higher in those receiving ART (552 [IQR, 410–691] cells/µL). Conversely, untreated PWH had a higher CD8 T-cell count (772 [IQR, 611–1073] cells/µL) than those receiving ART (727 [IQR, 552–953] cells/µL). Median log10 VL per mL of plasma for ART-naive early and chronically HIV-infected groups was 4.5 (IQR, 3.8–5.0) and 5.1 (IQR, 4.4–5.5), respectively. PWH receiving ART for a median of 13.6 (IQR, 1.6–17.8) years had suppressed viremia of <50 (1.7 log10) copies/mL (Table 1).
Plasma REG3α Levels Were Elevated in HIV-Infected Participants and Decreased With ART
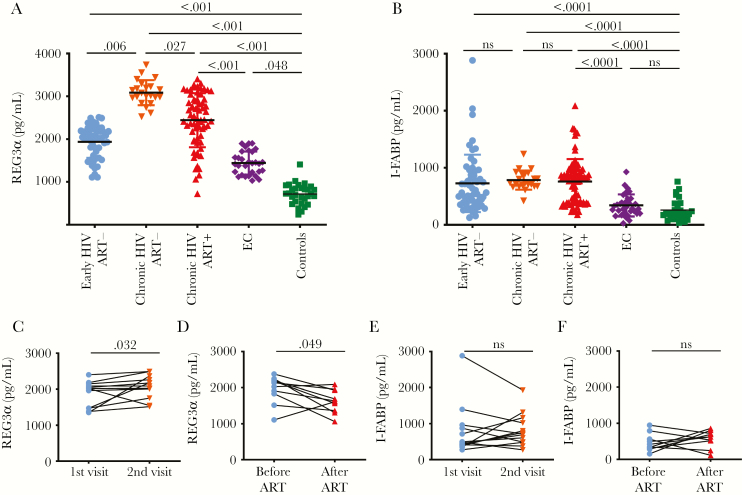
Cross-sectional analysis showed higher plasma levels of REG3α during early infection (mean 1938 ± standard deviation 374 pg/mL), untreated chronic infection (3084 ± 293 pg/mL), and ART-treated PWH (2441 ± 630 pg/mL) compared to controls (715 ± 243 pg/mL) (P < .0001 for all). Interestingly, REG3α levels were also elevated in ECs (1442 ± 270 pg/mL) compared with controls (P = .048). REG3α was higher in untreated chronic than untreated early HIV infection (P < .0001). Such values were also lower in treated chronic (P = .027) compared with untreated chronic HIV infection (Figure 1A). Longitudinal assessment of 12 early HIV-infected PWH not receiving ART showed an increase in REG3α levels from a median of 1878 ± 357 pg/mL to 2074 ± 328 pg/mL over a 24-month interval (Figure 1C; P = .032). One participant had a decrease in plasma REG3α. For this participant, CD4 count increased (719 then 881 cells/µL), and CD4:CD8 ratio remained elevated (0.83 vs 0.69) despite similar viremia at the 2 timepoints (4.15 vs 4.25 log10 copies/mL). This participant may have had a slower T-cell depletion and lower gut damage than other untreated participants [32, 33], mimicking the SIV-infection tolerance of sooty mangabeys [34, 35]. Conversely, 10 participants with early HIV infection who initiated ART during follow-up had decreased REG3α levels after 24 months (1952 ± 385 vs 1622 ± 318 pg/mL; P = .049) (Figure 1D). One participant had an increase in REG3α levels after 2 years on ART. This participant had an undetectable viremia (<1.7 log10 copies/mL) at the time of the second sampling and his CD4 count was increased (289 vs 557 cells/µL). However, we did not collected information on potential diarrhea, colitis, or any digestive illness for this participant at the second timepoint.
Figure 1.
Plasma levels of regenerating islet-derived protein 3α (REG3α) were elevated over the course of human immunodeficiency virus (HIV) infection. A, Plasma REG3α levels during early and chronic infection compared to elite controllers (ECs) and uninfected controls. Early HIV without antiretroviral therapy (ART–) (n = 51), chronic HIV without ART (n = 22), chronic HIV with ART (ART+; n = 66), ECs (n = 30), and controls (n = 30), Kruskal–Wallis test. B, Plasma intestinal fatty acid binding protein (I-FABP) levels in early HIV infection without ART (n = 56), chronic HIV without ART (n = 22), chronic HIV with ART (n = 71), ECs (n = 30), and controls (n = 30), Kruskal–Wallis test. Longitudinal analysis showed that plasma levels of REG3α (C) but not I-FABP levels (E) increased over 24 months in people living with HIV (PWH) without ART (n = 12), Wilcoxon test. Longitudinal analysis showed that plasma levels of REG3α (D) but not I-FABP (F) decreased in PWH after 24 months on ART (n = 10), Wilcoxon test. ns, not significant.
Nonparametric analyses showed that neither duration of ART nor the time to ART initiation had an influence on REG3α levels in ART-treated, chronically HIV infected participants (r = –0.07, P = .65 and r = 0.02, P = .89, respectively; data not shown). Multivariate analysis showed that elevated REG3α levels among PWH were independent of sex, age, and CD4 and CD8 T-cell counts (data not shown). There was no association between REG3α levels and serum levels of the pancreatic enzymes lipase and amylase (data not shown).
Plasma levels of I-FABP were elevated in early, chronic ART-naive and chronic ART-treated PWH compared to controls (P < .0001 for all 3 comparisons) (Figure 1B). ECs did not have elevated plasma levels of I-FABP compared with controls (P > .99). As opposed to REG3α, no differences in plasma levels of I-FABP were observed between the different HIV-infected groups, including early, chronic ART-naive and chronic ART-treated PWH. Prospective analysis demonstrated that plasma levels of I-FABP did not change significantly after 2 years in PWH who initiated ART and those who did not (Figure 1E and 1F).
Plasma Levels of REG3α Correlated With Markers of HIV Disease Progression
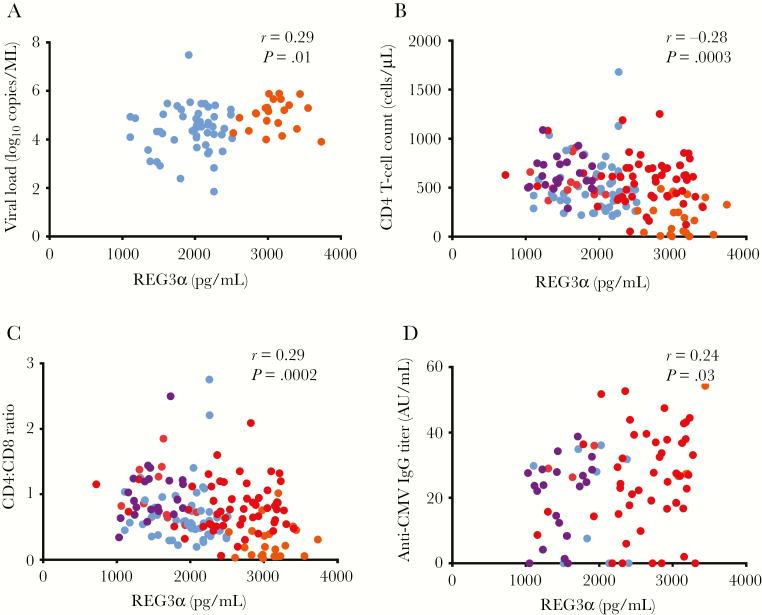
REG3α levels correlated positively with HIV plasma VL (r = 0.29, P = .013) (Figure 2A) in both untreated early and chronic HIV infection. Plasma levels of REG3α also inversely correlated with CD4 T-cell count (r = –0.28, P = .0003) (Figure 2B) and CD4:CD8 ratio (r = –0.29, P = .0002) in PWH (Figure 2C). No correlations were observed between REG3α levels and CD8 T-cell count (r = –0.006, P = .94) (Table 2). Plasma levels of I-FABP did not correlate with these markers (Table 2). REG3α plasma levels were also associated with total plasma IgG and with anti-CMV IgG titer in PWH (r = 0.24, P = .03) (Table 2 and Figure 2D, respectively).
Figure 2.
Plasma levels of regenerating islet-derived protein 3α (REG3α) correlated with markers of disease progression. A, Plasma REG3α levels correlated with human immunodeficiency virus (HIV) viral load in participants with early HIV and chronic HIV without ART (n = 72). B, Plasma REG3α inversely correlated with CD4 T-cell count (B) and CD4:CD8 ratio (C) in most participants (n = 161). D, Plasma REG3α was associated with anti-cytomegalovirus (CMV) immunoglobulin G (IgG) titer in people living with HIV (n = 82). Spearman test was used for correlations. Light blue indicates early HIV infection, orange indicates chronic HIV infection without ART, red indicates chronic HIV infection with ART, and purple indicates elite controllers.
Table 2.
Correlation Between Plasma Levels of Regenerating Islet-Derived Protein 3α or Intestinal Fatty Acid Binding Protein and Markers of Disease Progression, Microbial Translocation, and Inflammation in People Living With Human Immunodeficiency Virus (HIV) in Early and Chronic HIV Infection
| Correlation With Plasma Levels in PWH | ||
|---|---|---|
| Parameter | REG3α | I-FABP |
| CD4 T-cell count | r = –0.28 | r = –0.07 |
| P = .0003 | P = .39 | |
| n = 161 | n = 160 | |
| CD8 T-cell count | r = –0.006 | r = 0.11 |
| P = .94 | P = .16 | |
| n = 161 | n = 160 | |
| CD4:CD8 ratio | r = –0.29 | r = –0.35 |
| P = .0002 | P = .66 | |
| n = 161 | n = 160 | |
| Viral load | r = 0.29 | r = 0.0015 |
| P = .0134 | P = .99 | |
| n = 72 | n = 72 | |
| LPS | r = 0.24 | r = 0.047 |
| P = .005 | P = .56 | |
| n = 139 | n = 160 | |
| β-d-glucan | r = 0.19 | r = 0.11 |
| P = .03 | P = .17 | |
| n = 139 | n = 160 | |
| Soluble CD14 | r = 0.37 | r = –0.0012 |
| P = .0013 | P = .99 | |
| n = 74 | n = 83 | |
| Tryptophan | r = –0.41 | r = –0.031 |
| P = .0001 | P = .78 | |
| n = 81 | n = 87 | |
| Kynurenine | r = 0.30 | r = 0.12 |
| P = .0056 | P = .26 | |
| n = 81 | n = 88 | |
| Kynurenine/tryptophan ratio | r = 0.39 | r = 0.12 |
| P = .0003 | P = .28 | |
| n = 81 | n = 87 | |
| IL-6 | r = 0.53 | r = 0.11 |
| P < .0001 | P = .17 | |
| n = 139 | n = 160 | |
| IL-8 | r = 0.51 | r = 0.17 |
| P < .0001 | P = .035 | |
| n = 190 | n = 160 | |
| TNF-α | r = –0.006 | r = 0.13 |
| P = .94 | P = .10 | |
| n = 139 | n = 160 | |
| Total IgG | r = 0.44 | r = 0.16 |
| P = .0019 | P = .27 | |
| n = 47 | n = 49 | |
| Total IgM | r = 0.2 | r = –0.081 |
| P = .18 | P = .58 | |
| n = 47 | n = 49 | |
| Total IgA | r = –0.12 | r = 0.005 |
| P = .43 | P = .97 | |
| n = 50 | n = 52 | |
Values in boldface indicate P values <.05.
Abbreviations: β-d-glucan (1→3)-β-d-glucan; I-FABP, intestinal fatty acid binding protein; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IL, interleukin; LPS, lipopolysaccharide; PWH, people living with human immunodeficiency virus; REG3α, regenerating islet-derived protein 3α; TNF, tumor necrosis factor.
REG3α Levels Were Associated With Markers of Epithelial Gut Damage and Microbial Translocation
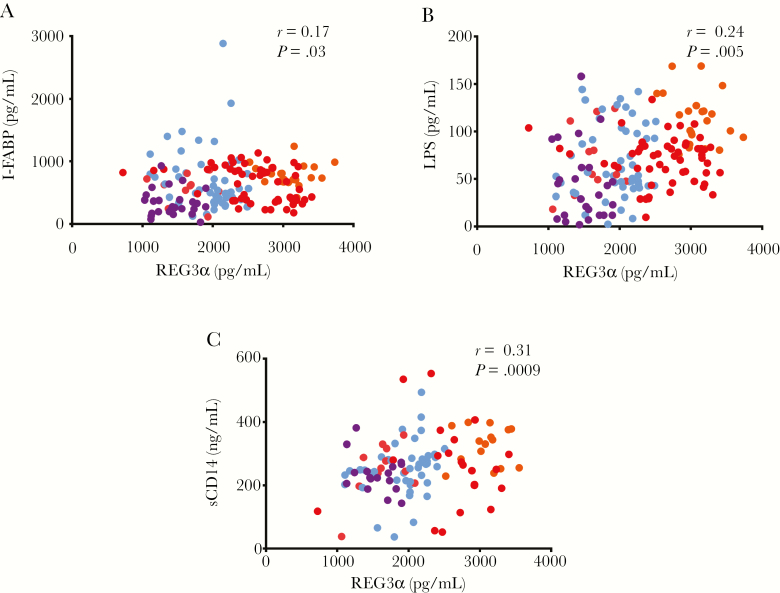
Plasma REG3α levels were weakly correlated with plasma levels of I-FABP in PWH (r = 0.17, P = .029) (Figure 3A). Loss of barrier integrity is implicated in the translocation of microbial products from the gut lumen into the blood [1, 12, 14, 15]. Indeed, plasma levels of LPS, a validated marker of bacterial translocation [1], correlated with REG3α levels in HIV progressors (r = 0.24, P = .005) (Figure 3B), but not in ART-treated chronic PWH only (Table 3). In addition, REG3α levels correlated with sCD14, a marker of myeloid cell activation following LPS stimulation (r = 0.31, P = .0009; Figure 3C). Plasma levels of β-d-glucan, a marker of fungal translocation [15], also correlated with REG3α levels (r = 0.19, P = .028) (Table 2), including in ART-treated chronic PWH (Table 3). Plasma levels of I-FABP did not correlate with these markers of microbial translocation (Table 2), with the exception of β-d-glucan plasma levels among ART-treated people living with chronic HIV (Table 3).
Figure 3.
Plasma levels of regenerating islet-derived protein 3α (REG3α) were associated with markers of epithelial gut damage and microbial translocation. A, Plasma levels of REG3α correlated with plasma intestinal fatty acid binding protein (I-FABP) levels in all people living with human immunodeficiency virus (PWH; n = 165). B, Plasma levels of REG3α were correlated with plasma levels of lipopolysaccharide (LPS; n = 163). C, Plasma levels of REG3α correlated with plasma levels of soluble CD14 (sCD14) in PWH (n = 109). Spearman test was used for correlations. Light blue indicates early human immunodeficiency virus (HIV) infection, orange indicates chronic HIV infection without antiretroviral therapy (ART), red indicates chronic HIV infection with ART, and purple indicates elite controllers.
Table 3.
Correlation Between Plasma Levels of Regenerating Islet-Derived Protein 3α or Intestinal Fatty Acid Binding Protein and Markers of Disease Progression, Microbial Translocation, and Inflammation in Antiretroviral Therapy–Treated People Living With Human Immunodeficiency Virus in Chronic Infection
| Correlation With Plasma Levels in ART-Treated PWH With Chronic Infection | |||
|---|---|---|---|
| Parameter | REG3α | I-FABP | |
| CD4 T-cell count | r = –0.015 | r = –0.009 | |
| P = .90 | P = .94 | ||
| n = 66 | n = 66 | ||
| CD8 T-cell count | r = –0.022 | r = –0.062 | |
| P = .86 | P = .62 | ||
| n = 66 | n = 66 | ||
| CD4:CD8 ratio | r = –0.069 | r = 0.016 | |
| P = .58 | P = .89 | ||
| n = 66 | n = 66 | ||
| LPS | r = –0.035 | r = –0.039 | |
| P = .78 | P = .75 | ||
| n = 66 | n = 66 | ||
| β-d-glucan | r = 0.25 | r = 0.39 | |
| P = .04 | P = .0011 | ||
| n = 66 | n = 66 | ||
| Soluble CD14 | r = –0.22 | r = 0.035 | |
| P = .48 | P = .92 | ||
| n = 12 | n = 12 | ||
| Tryptophan | r = –0.46 | r = –0.33 | |
| P = .02 | P = .12 | ||
| n = 24 | n = 24 | ||
| Kynurenine | r = 0.76 | r = 0.48 | |
| P = .73 | P = .017 | ||
| n = 24 | n = 24 | ||
| Kynurenine/tryptophan ratio | r = 0.4 | r = 0.56 | |
| P = .05 | P = .0045 | ||
| n = 24 | n = 24 | ||
| IL-6 | r = 0.29 | r = 0.044 | |
| P = .01 | P = .72 | ||
| n = 66 | n = 66 | ||
| IL-8 | r = 0.32 | r = 0.22 | |
| P = .008 | P = .06 | ||
| n = 66 | n = 66 | ||
| TNF-α | r = –0.035 | r = 0.085 | |
| P = .78 | P = .50 | ||
| n = 66 | n = 66 | ||
Values in boldface indicate P values <.05.
Abbreviations: β-d-glucan (1→3)-β-d-glucan; ART, antiretroviral therapy; I-FABP, intestinal fatty acid binding protein; IL, interleukin; LPS, lipopolysaccharide; PWH, people living with human immunodeficiency virus; REG3α, regenerating islet-derived protein 3α; TNF, tumor necrosis factor.
REG3α Levels Correlated With Markers of Systemic Immune Activation
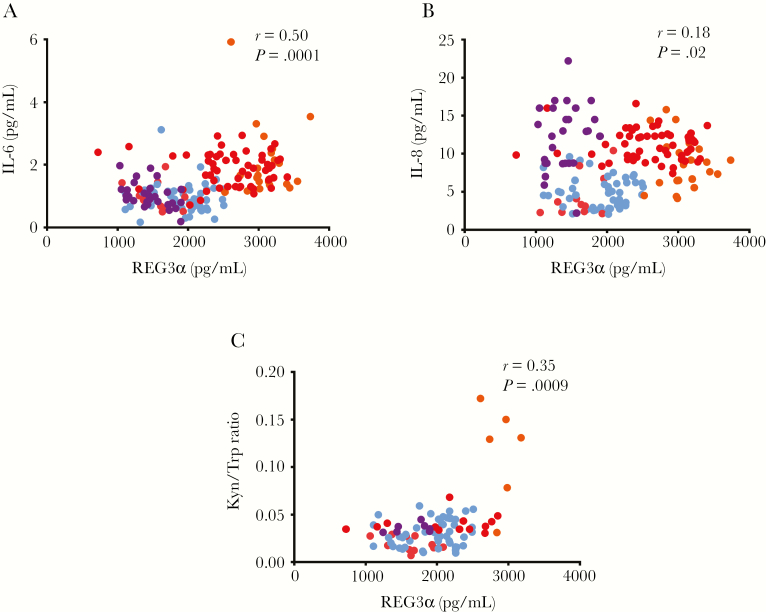
As gut permeability allows for increased microbial translocation, intestinal damage has been associated with increased inflammation in PWH [1, 15]. In our study participants, plasma levels of IL-6 and IL-8, which have been linked with the risk of developing non-AIDS comorbidities [2], were strongly correlated with plasma levels of REG3α in early and chronically HIV-infected participants (r = 0.50, P < .0001 [IL-6] and r = 0.18, P < .02 [IL-8]) (Figure 4A and 4B). Such correlations were maintained in ART-treated chronic PWH (Table 3) Plasma levels of CXCL13, a marker of immune activation in PWH, were also associated with REG3α (r = 0.16, P = .04; data not shown) [31]. Conversely, plasma levels of TNF-α were not associated with plasma levels of REG3α (Table 2). A trend was observed between plasma levels of REG3α and IL-22 (r = 0.21, P = .06; data not shown), a cytokine involved in the immune response against bacterial pathogens in epithelial cells [36]. Plasma levels of REG3α also correlated with the innate activation marker IDO-1, whose enzymatic activity was measured by the kynurenine-to-tryptophan ratio in plasma of PWH in both early and chronic HIV infection (r = 0.35, P = .0009; Figure 4C). The association of REG3α levels with markers of inflammation and IDO-1 activity was independent of sex, age, and CD4 and CD8 T-cell counts. Importantly, such correlations were not detected with plasma levels of I-FABP (Table 2).
Figure 4.
Plasma levels of regenerating islet-derived protein 3α (REG3α) were associated with markers of myeloid and lymphoid activation. Plasma levels of REG3α were correlated with plasma levels of interleukin 6 (IL-6; A) and interleukin 8 (IL-8; B) (n = 168). Plasma levels of REG3α correlated with indoleamine-2,3-dioxygenase (IDO-1) activity as REG3α levels were positively associated with the kynurenine-to-tryptophan (Kyn/Trp) ratio (C) in a subset of people living with human immunodeficiency virus (HIV) (n = 88). Spearman test was used for correlations. Light blue indicates early HIV infection, orange indicates chronic HIV infection without antiretroviral therapy (ART), red indicates chronic HIV infection with ART, and purple indicates elite controllers.
Last, we observed that plasma levels of REG3α levels were associated with the percentage of activated HLA-DR+CD38+CD4 (r = 0.46, P = .048; Supplementary Figure 1A) and CD8 T-cells (r = 0.66, P = .002; Supplementary Figure 1B). Conversely, plasma levels of I-FABP were not associated with percentage of activated CD4 (r = 0.20, P = .39; data not shown) nor CD8 T cells (r = 0.04, P = .87; data not shown).
DISCUSSION
REG3α has been previously validated as a marker of gut damage in IBDs and GVHD as it is solely produced in the intestine [25–27, 37]. As PWH present with intestinal abnormalities even after long-term ART, we assessed their plasma levels of REG3α. To our knowledge, we are the first to report elevated circulating REG3α in untreated and ART-treated PWH. Plasma levels of REG3α were increased in PWH in chronic infection compared to those in early infection. Initiation of ART was associated with a decrease without normalization of plasma levels of REG3α. In contrast to I-FABP, plasma levels of REG3α were significantly higher in ECs compared with controls. Furthermore, plasma levels of REG3α were associated with markers of HIV disease progression and microbial translocation in untreated PWH, and with markers of systemic inflammation and T-cell activation in all PWH.
REG3α is a member of a family of antimicrobial peptides secreted by Paneth cells in the crypts of the intestinal epithelium. Gut antimicrobial peptides keep pathogens and commensal microbes at bay from the mucosa [38]. REG3α acts as a C-type lectin by binding to peptidoglycan from gram-positive bacteria, forming a pore that in turn kills bacteria [39]. REG3α is exclusively secreted into the intestinal lumen and thus its presence in the lamina propria is a marker of gut damage, allowing for its translocation into the blood. Hence, elevated plasma REG3α levels are an indicator of gut epithelium integrity. Increased gut damage and circulating levels of REG3α were found in people with Crohn disease, ulcerative colitis, and celiac disease, but not in people with irritable bowel syndrome [25].
As PWH, including those on long-term ART, have increased gut damage, they also experience increased microbial translocation and chronic inflammation. Because routine access to gut tissue samples remains challenging [16], methods of assessing gut damage in PWH rely on plasma/serum level markers. Existing markers such as soluble suppressor of tumorigenicity 2 assesses the degree of inflammation in any type of epithelium and were only elevated during the early phase of the infection in PWH [8]. Research studies commonly utilize circulating I-FABP as a measure of enterocyte cell death and turnover [11, 18]. Although circulating I-FABP is a reliable measure of the level of enterocyte damage, it yields little information concerning the degree of intestinal permeability prior to or after enterocyte lysis. In contrast, during homeostasis, REG3α is constitutively secreted into the gut lumen with very small quantities translocating into systemic circulation. However, upon gut damage, REG3α translocates into systemic circulation. Thus, circulating REG3α levels reflect the degree of gut damage independent of enterocyte cell death.
Plasma levels of REG3α were more elevated in chronic vs early HIV infection. Moreover, our longitudinal analyses demonstrated an increase of REG3α levels in the absence of ART. These findings are consistent with our knowledge of the degree of gut damage in PWH. In contrast, no significant differences in I-FABP levels were detected between the early and chronic phase of HIV infection in our cross-sectional nor in the longitudinal analysis. Measuring gut damage with REG3α compared to I-FABP levels allows for a better identification of participants in early or chronic HIV infection as well as ECs from controls. In addition, stronger correlations were detected between plasma levels of REG3α with most validated markers of disease progression, microbial translocation, and inflammation.
This article is the first to report circulating levels of REG3α in ECs to be significantly higher than controls while remaining lower than HIV progressors. This was expected as ECs display reduced epithelial gut damage compared with HIV-infected progressors [11]. However, persistence of chronic inflammation and elevated plasma levels of sCD14 suggest that, similar to HIV-infected progressors, ECs also present with chronic microbial translocation and inflammation [40]. Indeed, increased gut damage may explain the higher frequency of non-AIDS comorbidities like cardiovascular diseases in ECs compared to controls [41].
We and others have previously shown an association between loss of barrier integrity, gut damage, and translocation of bacterial and fungal products into the systemic circulation [1, 12, 14, 15]. Indeed, study findings demonstrated that circulating levels of REG3α correlated with plasma levels of LPS and β-d-glucan levels in PWH. As increased gut damage allows the passage of microbial products into the circulation, it also contributes to systemic inflammation and immune activation. We observed that REG3α correlated with plasma levels of proinflammatory cytokines such as IL-6 and IL-8, and the percentage of activated CD4 and CD8 T cells. REG3α levels were also associated with total IgG but not IgM or IgA plasma levels, suggesting a link between gut damage and B-cell activation. Moreover, IDO-1 activity, measured by plasma tryptophan-to-kynurenine ratio, was strongly correlated with REG3α levels. As IDO-1 activity is linked with dysbiosis and microbial translocation during HIV infection, this further strengthens the association between REG3α and gut inflammation in PWH [10, 42]. IDO-1 activity is a marker of activated innate immune cells such as monocytes and macrophages and has been shown to predict cardiovascular diseases [43]. Moreover, anti-CMV IgG titer was associated with inflammation and mortality in HIV-uninfected population [44]. In line with our previous findings showing an association between CMV coinfection and elevated gut damage in PWH [45, 46], we found an association between plasma REG3α and anti-CMV IgG titer.
Conversely to plasma levels of I-FABP, REG3α were associated with levels of CD4 and CD8 T-cell activation, which have been reported to be a predictor of HIV disease progression independently of plasma VL [2].
We acknowledge that our study presents some limitations as we did not assess gut microbiota composition in our study participants. Elevated REG3α production in the gut lumen would also be associated with shifts in microbiota composition resulting from the killing of certain bacteria as seen in a mouse model of colitis [47]. However, the causative role of REG3α in microbiota modification in PWH has yet to be explored. In addition, although we accounted for several factors such as usage of antibiotics and CMV coinfection, we did not collect information on alcohol consumption in our participants, which might play a role in gut damage. In this study, we did not assess the predictive value of REG3α elevation for the risk of development of non-AIDS comorbidities, as clinical outcomes were rare. Last, gut tissue expression of REG3α needs to be studied to confirm its role and value as a marker of gut damage in PWH [37].
CONCLUSIONS
Compared to I-FABP, REG3α plasma levels were able to identify participants in early or chronic HIV infection as well as those receiving or not receiving ART, and ECs. In addition, REG3α presented stronger correlations with several validated makers of HIV disease progression and inflammation.
As gut damage and microbial translocation are associated with inflammation and non-AIDS comorbidities [2, 48], robust markers of epithelial gut damage are warranted to provide better care for PWH. We showed that plasma levels of the C-type lectin REG3α were elevated in PWH. Measuring REG3α levels may contribute to the assessment of the risk of developing non-AIDS comorbidities in PWH and provide a useful marker to evaluate therapeutic interventions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, 21–24 July 2019.
Acknowledgments. The authors are highly grateful to the study participants for their contribution. They thank Angie Massicotte, Josée Girouard, and Cynthia Dion for study coordination and assistance; Jing Ouyang and Vikram Mehraj for helpful discussion; and Mario Legault, Stéphanie Matte, Olfa Debbeche, and Sylla Mohamed for coordinating the Montreal Primary HIV Infection Study and the Canadian HIV and Aging Cohort Study. The authors are also thankful to the following physicians for their contributions: C. Milne, S. Lavoie, J. Friedman, M. Duchastel, F. Villielm, F. Asselin, M. Boissonnault, P. J. Maziade, S. Lavoie, M. Milne, N. Z. Miaki., and M. E. Thériault (Clinique médicale l’Actuel); B. Lessard, M. A. Charron, S. Dufresne, M. E. Turgeon, S. Vézina, E. Huchet, J. P. Kerba, M. Poliquin, S. Poulin, P. Rochette, P. Junod, D. Longpré, R. Pilarski, E. Sasseville, L. Charest, A. Hamel, A. Cloutier-Blais, S. Massoud, F. Chano, and B. Trottier (Clinique médicale urbaine du Quartier Latin); L. Labrecque, C. Fortin, V. Hal-Gagne, M. Munoz, B. Deligne, V. Martel-Laferrière, B. Trottier, and M. E. Goyer (Unité Hospitalière de Recherche, d’Enseignement et de Soins sur le Sida Centre Hospitalier de l’Université de Montréal Hôtel-Dieu and Notre-Dame); and M. Teltscher, A. de Pokomandy, J. Cox, E. Beauchamp, M. Klein, and L. P. Haraoui (Chronic Viral Illness Service, McGill University Health Centre).
Author contributions. S. I. performed the experiments, analyzed the data, wrote the first draft, and finalized the manuscript. R. R. performed the experiments, analyzed the data, and revised the final draft of the manuscript., F. P. D., J. L., B. F., N. K., I. K., M-A. J., P. A., N. F. B., M. S., and P. L. contributed to the experiments, data analysis, and critical review of final drafts of the manuscript. J.-P. R. designed the study, contributed to data analysis, and critically reviewed the first and final drafts of the manuscript. C. C., B. L., M. D., C. T., and P. A. all contributed to recruitment and follow-up of study participants and critically reviewed the manuscript. All authors have read and approved the contents of this manuscript.
Financial support This work was supported by the Fonds de la Recherche Québec-Santé, Réseau SIDA/Maladies Infectieuses and Thérapie Cellulaire; the Canadian Institutes of Health Research (CIHR; grant numbers MOP 103230, 154051, and PJT 166049); the Vaccines and Immunotherapies Core of the CIHR Canadian HIV Trials Network (grant number CTN 257); and the Canadian HIV Cure Enterprise 1.0 and 2.0 Team supported by the CIHR (grant numbers HIG133050 and HB2-164064). The Long-Term Non-Progressors cohort is supported by the CIHR (grant numbers MOP93770). The Canadian HIV and Aging Cohort is supported by the CIHR (grant numbers TCO-125276 and HAL-157985). S. I. is a postdoctoral fellow supported by the William Turner research fellowship. R. R. is an undergraduate student supported by the H. Grenville Smith Studentship. M.-A. J. is holder of a Tier 2 Canada Research Chair in immunovirology. J.-P. R. is the holder of the Louis Lowenstein Chair in Hematology and Oncology, McGill University, and the William Turner award holder from the McGill University Health Centre.
Potential conflicts of interest. C. T. has received grants and personal fees from Merck, Gilead, and ViiV, as well as personal fees from Theratechnologie. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Montreal Primary HIV Infection Study, the Canadian Cohort of HIV+ Slow Progressors, and the Canadian HIV and Aging Cohort Groups:
C Milne, S Lavoie, J Friedman, M Duchastel, F Villielm, F Asselin, M Boissonnault, P J Maziade, S Lavoie, M Milne, N Z Miaki, M E Thériault, B Lessard, M A Charron, S Dufresne, M E Turgeon, S Vézina, E Huchet, J P Kerba, M Poliquin, S Poulin, P Rochette, P Junod, D Longpré, R Pilarski, E Sasseville, L Charest, A Hamel, A Cloutier-Blais, S Massoud, F Chano, B Trottier, L Labrecque, C Fortin, V Hal-Gagne, M Munoz, B Deligne, V Martel-Laferrière, B Trottier, M E Goyer, M Teltscher, A de Pokomandy, J Cox, E Beauchamp, M Klein, and L P Haraoui
References
- 1. Brenchley JM, Price DA, Schacker TW, et al. . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 2. Hsu DC, Sereti I. Serious non-AIDS events: therapeutic targets of immune activation and chronic inflammation in HIV infection. Drugs 2016; 76:533–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tenorio AR, Zheng Y, Bosch RJ, et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Batman PA, Miller AR, Forster SM, Harris JR, Pinching AJ, Griffin GE. Jejunal enteropathy associated with human immunodeficiency virus infection: quantitative histology. J Clin Pathol 1989; 42:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batman PA, Kotler DP, Kapembwa MS, et al. . HIV enteropathy: crypt stem and transit cell hyperproliferation induces villous atrophy in HIV/Microsporidia-infected jejunal mucosa. AIDS 2007; 21:433–9. [DOI] [PubMed] [Google Scholar]
- 6. Heise C, Dandekar S, Kumar P, Duplantier R, Donovan RM, Halsted CH. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology 1991; 100:1521–7. [DOI] [PubMed] [Google Scholar]
- 7. Hensley-McBain T, Berard AR, Manuzak JA, et al. . Intestinal damage precedes mucosal immune dysfunction in SIV infection. Mucosal Immunol 2018; 11:1429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehraj V, Jenabian MA, Ponte R, et al. . Montreal Primary HIV Infection, the Canadian Long-Term Non-Progressors Study Groups The plasma levels of soluble ST2 as a marker of gut mucosal damage in early HIV infection. AIDS 2016; 30:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ponte R, Mehraj V, Ghali P, Couëdel-Courteille A, Cheynier R, Routy JP. Reversing gut damage in HIV infection: using non-human primate models to instruct clinical research. EBioMedicine 2016; 4:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenabian MA, El-Far M, Vyboh K, et al. . Montreal Primary infection and Slow Progressor Study Groups Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015; 212:355–66. [DOI] [PubMed] [Google Scholar]
- 11. Cheru LT, Park EA, Saylor CF, et al. . I-FABP is higher in people with chronic HIV than elite controllers, related to sugar and fatty acid intake and inversely related to body fat in people with HIV. Open Forum Infect Dis 2018; 5:ofy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramendra R, Isnard S, Mehraj V, et al. . Circulating LPS and (1→3)-β-D-glucan: a folie à deux contributing to HIV-associated immune activation. Front Immunol 2019; 10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klatt NR, Harris LD, Vinton CL, et al. . Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol 2010; 3:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Estes JD, Harris LD, Klatt NR, et al. . Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010; 6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehraj V, Ramendra R, Isnard S, et al. . Circulating (1→3)-beta-D-glucan is associated with immune activation during HIV infection [manuscript published online ahead of print 16 March 2019]. Clin Infect Dis 2019. doi:10.1093/cid/ciz212. [Google Scholar]
- 16. Mehraj V, Ghali P, Ramendra R, et al. . The evaluation of risk-benefit ratio for gut tissue sampling in HIV cure research. J Virus Erad 2017; 3:212–7. [PMC free article] [PubMed] [Google Scholar]
- 17. Al-Saffar AK, Meijer CH, Gannavarapu VR, et al. . Parallel changes in Harvey-Bradshaw index, TNFα, and intestinal fatty acid binding protein in response to infliximab in Crohn’s disease. Gastroenterol Res Pract 2017; 2017:1745918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adriaanse MP, Tack GJ, Passos VL, et al. . Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther 2013; 37:482–90. [DOI] [PubMed] [Google Scholar]
- 19. El Kamari V, Sattar A, Mccomsey GA. Brief report: gut structural damage: an ongoing process in chronically untreated HIV infection. J Acquir Immune Defic Syndr 2019; 80:242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Kamari V, Moser C, Hileman CO, et al. . Lower pretreatment gut integrity is independently associated with fat gain on antiretroviral therapy. Clin Infect Dis 2019; 68:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sereti I, Krebs SJ, Phanuphak N, et al. . RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 Protocol Teams Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017; 64:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steele AK, Lee EJ, Vestal B, et al. . Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PLoS One 2014; 9:e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9:356–68. [DOI] [PubMed] [Google Scholar]
- 24. Shin JH, Seeley RJ. Reg3 proteins as gut hormones? Endocrinology 2019; 160:1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marafini I, Di Sabatino A, Zorzi F, et al. . Serum regenerating islet-derived 3-alpha is a biomarker of mucosal enteropathies. Aliment Pharmacol Ther 2014; 40:974–81. [DOI] [PubMed] [Google Scholar]
- 26. Ferrara JL, Harris AC, Greenson JK, et al. . Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood 2011; 118:6702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao D, Kim YH, Jeong S, et al. . Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest 2018; 128:4970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durand M, Chartrand-Lefebvre C, Baril JG, et al. . Investigators of the Canadian HIV and Aging Cohort Study The Canadian HIV and Aging Cohort Study—determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: rationale and study protocol. BMC Infect Dis 2017; 17:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehraj V, Cox J, Lebouche B, et al. . Socio-economic status and time trends associated with early ART initiation following primary HIV infection in Montreal, Canada: 1996 to 2015. J Int AIDS Soc 2018; 21. doi:10.1002/jia2.25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Department of Health and Human Services/National Institutes of Health. Acute HIV Infection and Early Diagnosis Research Program guidelines. 2019 https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 31. Mehraj V, Ramendra R, Isnard S, et al. . CXCL13 as a biomarker of immune activation during early and chronic HIV infection. Front Immunol 2019; 10:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodríguez B, Sethi AK, Cheruvu VK, et al. . Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 2006; 296:1498–506. [DOI] [PubMed] [Google Scholar]
- 33. Dion ML, Bordi R, Zeidan J, et al. . Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood 2007; 109:2912–20. [DOI] [PubMed] [Google Scholar]
- 34. McGary CS, Cervasi B, Chahroudi A, et al. . Increased stability and limited proliferation of CD4+ central memory T cells differentiate nonprogressive simian immunodeficiency virus (SIV) infection of sooty mangabeys from progressive SIV infection of rhesus macaques. J Virol 2014; 88:4533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muenchhoff M, Adland E, Karimanzira O, et al. . Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci Transl Med 2016; 8:358ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim CJ, Nazli A, Rojas OL, et al. . A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol 2012; 5:670–80. [DOI] [PubMed] [Google Scholar]
- 37. Ogawa H, Fukushima K, Naito H, et al. . Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis 2003; 9:162–70. [DOI] [PubMed] [Google Scholar]
- 38. Holly MK, Smith JG. Paneth cells during viral infection and pathogenesis. Viruses 2018; 10. doi:10.3390/v10050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mukherjee S, Zheng H, Derebe MG, et al. . Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014; 505:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li JZ, Arnold KB, Lo J, et al. . Differential levels of soluble inflammatory markers by human immunodeficiency virus controller status and demographics. Open Forum Infect Dis 2015; 2:ofu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crowell TA, Gebo KA, Blankson JN, et al. . HIV Research Network Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis 2015; 211:1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. . Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niinisalo P, Raitala A, Pertovaara M, et al. . Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest 2008; 68:767–70. [DOI] [PubMed] [Google Scholar]
- 44. Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172:363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog 2017; 13:e1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramendra R, Mehraj V, Isnard S, et al. . Elevated anti-CMV IgG titer is associated with microbial translocation in elite controllers and ART-treated people living with HIV [abstract TUPEB220]. In: International AIDS Society Conference, 2019. [Google Scholar]
- 47. Darnaud M, Dos Santos A, Gonzalez P, et al. . Enteric delivery of regenerating family member 3 alpha alters the intestinal microbiota and controls inflammation in mice with colitis. Gastroenterology 2018; 154:1009–23.e14. [DOI] [PubMed] [Google Scholar]
- 48. Hoenigl M, Moser C, Funderburg N, et al. . Soluble urokinase plasminogen activator receptor (suPAR) is predictive of non-AIDS events during antiretroviral therapy-mediated viral suppression [manuscript published online ahead of print 12 November 2018]. Clin Infect Dis 2018. doi:10.1093/cid/ciy966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.