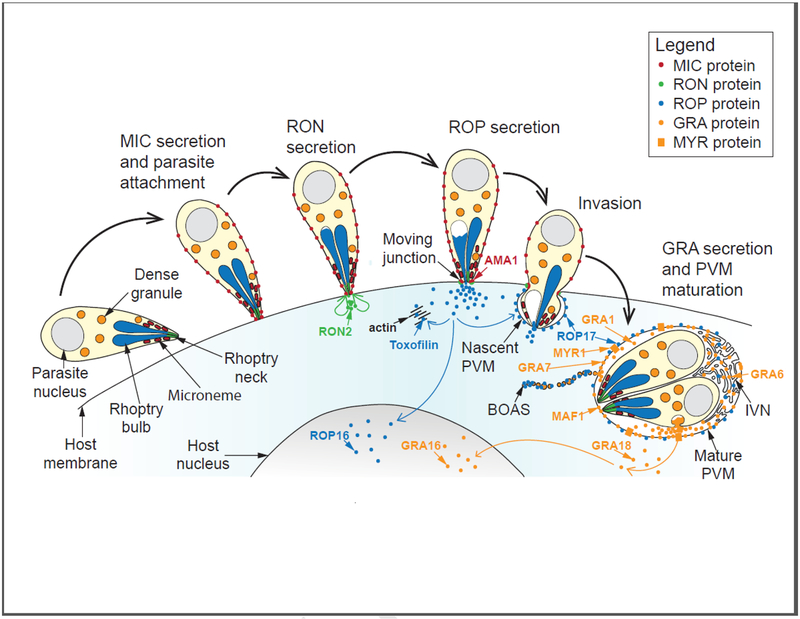
Figure 1:
An integrated model for effector protein secretion by Toxoplasma gondii tachyzoites into the host cell, illustrated in the context of Toxoplasma invasion. First, the micronemes secrete micronemal proteins (MICs) that are anchored in the parasite plasma membrane and mediate parasite attachment to the host cell (MIC secretion and parasite attachment). Upon strong parasite attachment to the host cell, the rhoptry organelle discharges in a manner simultaneous with parasite invasion, first introducing rhoptry neck proteins into the host cell (RON secretion) that go on to establish the moving junction, and subsequently rhoptry bulb proteins (ROP secretion) that either translocate to the host nucleus, associate with the nascent or fully formed parasitophorous vacuole membrane (PVM), or associate with cytosolic actin (i.e., toxofilin). Secretion of dense granule proteins (GRAs) into the host may also begin as early as during parasite invasion. Once the parasites have fully penetrated the host cell, PVM maturation occurs concomitantly with continued GRA secretion (GRA secretion and PVM maturation), and GRAs are either deposited into the PV space, PVM, or intravacuoloar network (IVN), or they are translocated across the PVM in a fashion dependent on several MYR proteins (orange rectangles), and end their journey in either the host cytosol or the host nucleus. Note that ROPs and GRAs are also found in structures called beads on a string (BOAS), vesicular-like entities of unknown function that may originate by blebbing off of the PVM. Selected parasite effector proteins that are examples of the different classes of proteins mentioned above are shown; i.e., the rhoptry proteins, RON2 (host plasma membrane), ROP16 (host nucleus), toxofilin (host cytosol), and ROP17 (PVM); and the dense granule proteins, GRA1 (PV space), GRA6 (IVN), GRA18 (host cytosol), GRA16 (host nucleus), GRA7 (PVM), and MAF1 and MYR1 (PVM).