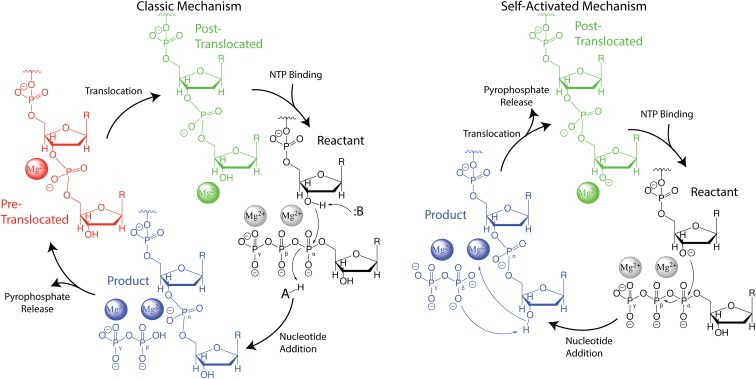
Fig. 1.
Mechanistic proposals for the nucleic acid elongation cycle by nucleic acid polymerases. Each of the states along the proposed cycles is colored differently (e.g., reactant, product, pre-translocated, and post-translocated). One of the key differences between the 2 mechanisms is the protonation state of the 3′-OH, which serves as the nucleophile for nucleotide addition. In the classic mechanism the 3′-OH is deprotonated by some base immediately prior to or concerted with nucleophilic attack. In the self-activated mechanism, the pyrophosphate leaving group deprotonates the 3′-OH, which leaves the 3′-O deprotonated in the post-translocated state, prior to binding of the next NTP. One of the Mg2+ ions is bound principally by the NTP and is thought to be released when pyrophosphate is released. Additionally, recent work indicated that a third Mg2+ plays some role in catalysis (11). We have omitted the third Mg2+ from this figure for clarity, but when appropriate, our simulations include it to explore its role in the reaction.