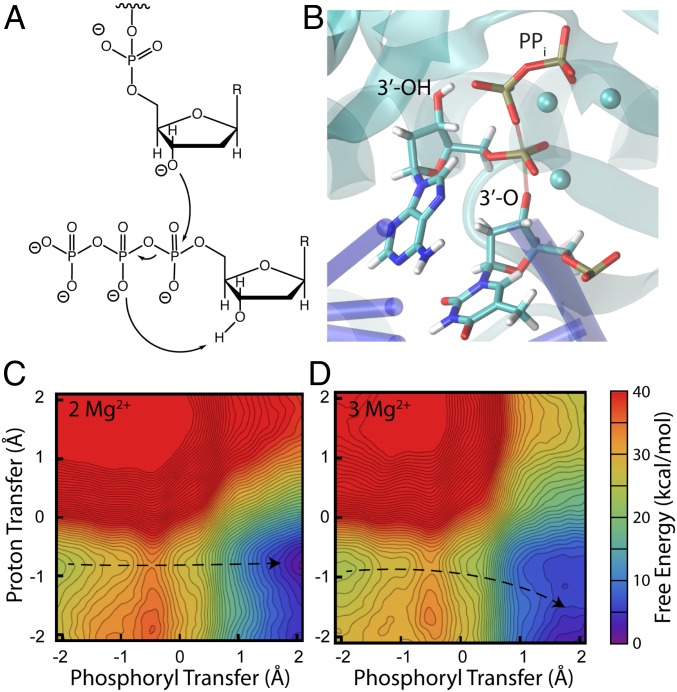
Fig. 3.
Mechanism (A), transition state structure (B), and free-energy surfaces for nucleotide addition in the self-activated mechanism with either 2 Mg2+ (C) or 3 Mg2 (D). The proximity of the pyrophosphate leaving group to the 3′-OH of the just-added nucleotide suggested that the pyrophosphate could deprotonate the 3′-OH (7). The phosphoryl transfer coordinate is defined as the difference in length of the breaking and forming O–P bonds, and the proton transfer coordinate is defined as the difference in length of the breaking and forming O–H bonds. The structure in B is representative of the transition state region for the reaction with three Mg2+, with the breaking and forming bonds shown as transparent. The third Mg2+ ion lowers the barrier for phosphoryl transfer (∆G‡ = 10.7 kcal/mol vs. 7.9 kcal/mol for 2 and 3 Mg2+). In neither case, though, is subsequent proton abstraction by the pyrophosphate a plausible next step; that process is steeply endergonic regardless of the number of metals. While there is a short hydrogen bond between the leaving group and the 3′-OH of the nucleotide being added, the leaving group cannot abstract that proton. We note that in these surfaces, as well as all others in the main text, the simulations sampled the entirety of the displayed reaction space; the free-energy scale was truncated at +40 kcal/mol for all surfaces for clarity of individual surfaces and to simplify comparisons among the various mechanisms. The dotted lines guide the eye along the minimum free-energy path from reactant to product; the transition state corresponds to the location of the maximum free energy along this minimum path.