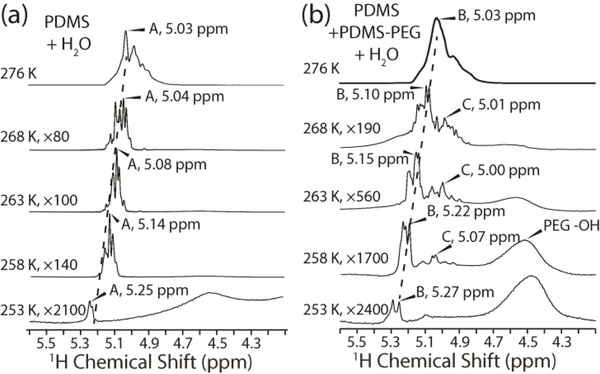
Figure 6.
1H spectra of PDMS+water (a) and PDMS+PDMS-PEG+water (b) collected between 276 K and 253 K. The dominant water peaks used for analysis of relaxation times are labeled A (a) and B (b). The PEG hydroxyl peak can be seen in (b) after magnifying the spectra greater than 500 fold. The peak labelled C in (b) is due to exchange of protons between water that is hydrogen bonded to PEG and the PEG hydroxyl on a time scale that is faster than the NMR experiments conducted.