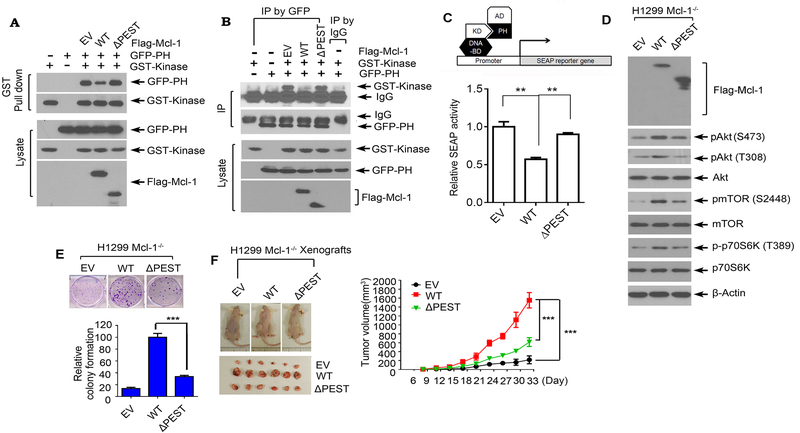
Figure 4.
The binding of Mcl-1 with Akt is essential for Mcl-1 to disrupt PH/KD interactions, upregulate Akt activity and promote tumor growth. A and B, GST-tagged kinase domain of Akt and GFP-tagged PH domain of Akt were co-transfected with Flag-tagged WT Mcl-1 or ΔPEST Mcl-1 mutant into H1299 cells, followed by GST pull down (A), or co-IP using GFP antibody (B), and Western blot using GST or GFP antibody. C, Interaction between the kinase domain and PH domain in the absence or presence of WT Mcl-1 or ΔPEST was measured in a mammalian two-hybrid system. D and E, H1299 Mcl-1−/− cells were transfected with empty vector (EV), Flag-tagged WT Mcl-1 or ΔPEST Mcl-1 mutant, followed by analysis of pAkt, pmTOR and p-p70 S6K by Western blot (D) and colony formation assay (E). Data represent the mean ± SD. ***P < 0.001, by 2-tailed t test. F, The same number (3×106) of H1299 Mcl-1−/− cells expressing EV, Flag-tagged WT Mcl-1 or ΔPEST mutant were injected into subcutaneous tissue in the flank region of nude mice to generate lung cancer xenografts. Tumor volume was measured once every 3 days. After 33 days, the mice were sacrificed and the tumors were removed, photographed, and analyzed. Data represent the mean ± SD, n=6 mice per group. ***P < 0.001, by 2-tailed t test.