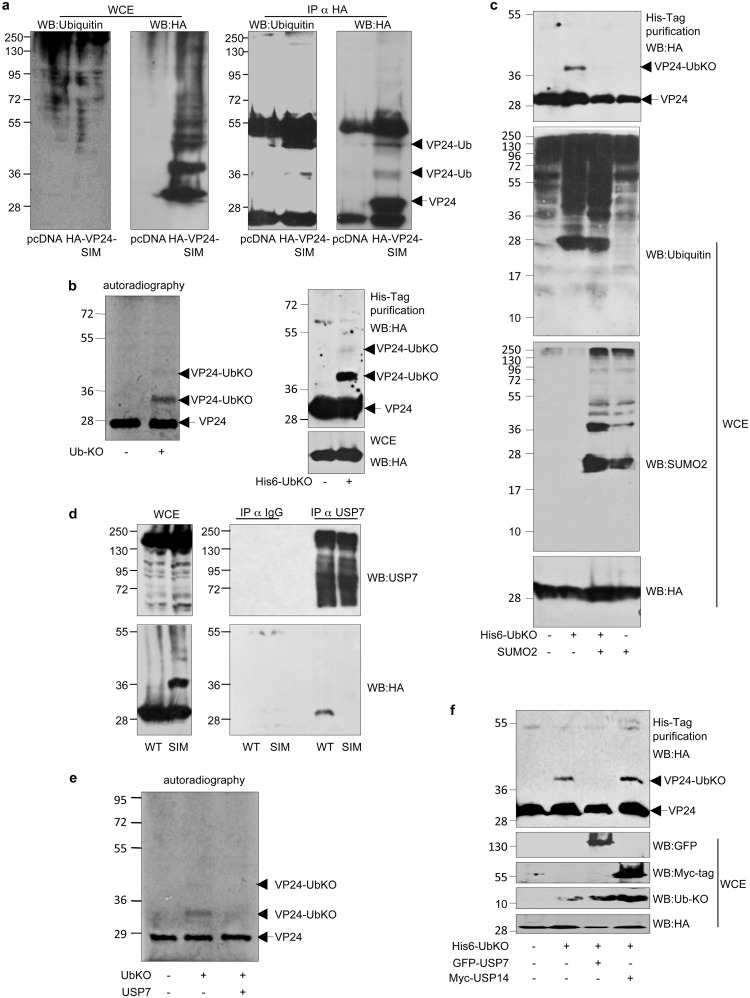
FIG 4.
Mutation of the SIM domain in VP24 inhibits its interaction with the ubiquitin-specific-processing protease 7 (USP7) and promotes its monoubiquitination. (a) HEK-293 cells were transfected with pcDNA or HA-VP24-SIM, and 24 h after transfection, protein extracts of transfected cells were immunoprecipitated using anti-HA antibody. Immunoprecipitated proteins were analyzed using anti-ubiquitin antibody. (b) In vitro ubiquitination assay with UbKO using 35S-methionine-labeled in vitro-translated VP24-WT protein (left panel). HEK-293 cells were cotransfected with VP24-WT and pcDNA or His6-ubiquitin KO. At 36 h after transfection, total cell extracts and histidine-purified proteins were analyzed by Western blotting using anti-HA antibody (right panel). (c) HEK-293 cells were cotransfected with VP24-WT and pcDNA, His6-ubiquitin KO and pcDNA, SUMO2 or SUMO2, and His6-ubiquitin KO, as indicated. At 36 h after transfection, total cell extracts and histidine-purified proteins were analyzed by Western blotting using anti-HA antibody. (d) HEK-293 cells were transfected with HA-VP24-WT or HA-VP24-SIM, and 24 h after transfection, protein extracts of transfected cells were immunoprecipitated using anti-USP7 antibody. Immunoprecipitated proteins were analyzed using anti-HA antibody. (e) Incubation of in vitro-ubiquitinated 35S-methionine-labeled in vitro-translated VP24-WT protein with UbKO in presence or absence of recombinant USP7. (f) HEK-293 cells were cotransfected with VP24-WT and pcDNA, His6-ubiquitin KO, His6-ubiquitin KO and GFP-USP7, or His6-ubiquitin KO and Myc-USP14. At 36 h after transfection, total cell extracts and histidine-purified proteins were analyzed by Western blotting using anti-HA antibody.