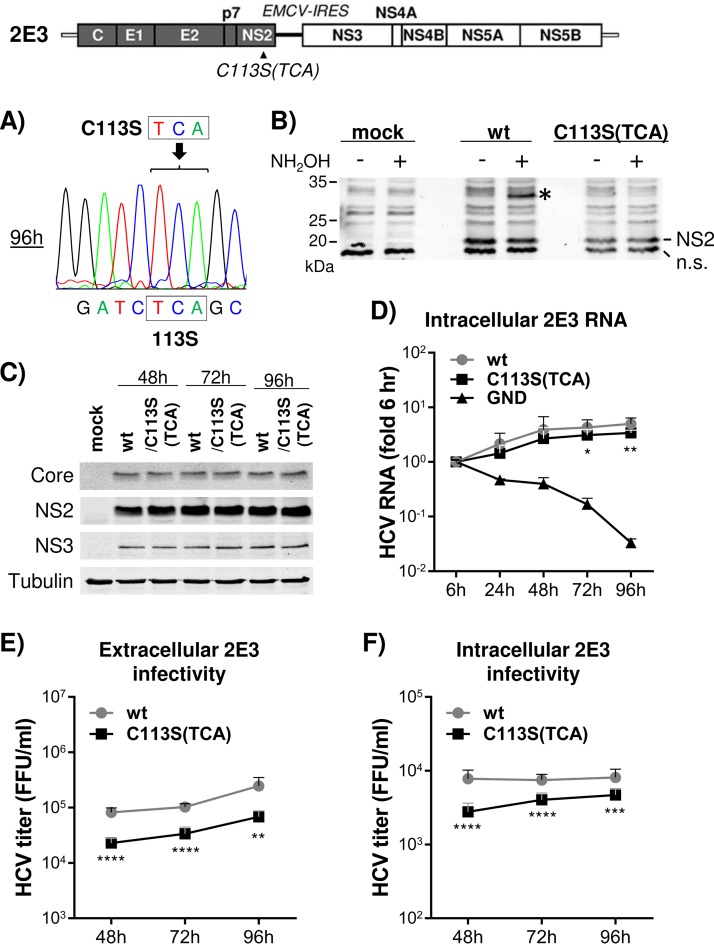
FIG 4.
C113S mutation-mediated inhibition of NS2 palmitoylation reduced both intra- and extracellular virus production. (Top) Schematic of 2E3 showing the location of the NS2/C113S(TCA) mutation. (A) Sequencing chromatogram showing maintenance of the NS2/C113S(TCA) mutation until 96 h postelectroporation of mutated 2E3 RNA. (B) Western blot detection of palmitoylated NS2 in cell lysates derived from wt or C113S(TCA) mutated 2E3 RNA replicating cells following APEGS assay. The PEGylated NS2 is indicated by an asterisk. (C) Western blot of HCV Core, NS2, NS3, and tubulin from cell lysates harvested at different time points postelectroporation of wt or NS2/C113S(TCA) mutated 2E3 RNA. (D) Results of quantitative RT (qRT)-PCR to detect HCV RNA present in cell lysates collected at different time points postelectroporation of 2E3 RNA with or without NS2/C113S(TCA) mutation. The graph represents the HCV RNA fold difference relative to the 6-h value. (E and F) Results of extracellular (E) and intracellular (F) viral titration using culture supernatants and cell lysates, respectively, harvested at different time points postelectroporation of wt or NS2/C113S mutated 2E3 RNA. (D, E, and F) The graphs show means with standard deviations from three independent experiments. The asterisks indicate statistically significant differences between paired values based on the Mann-Whitney test. ****, P < 0.00005; ***, P < 0.0005; **, P < 0.005; *, P < 0.05.