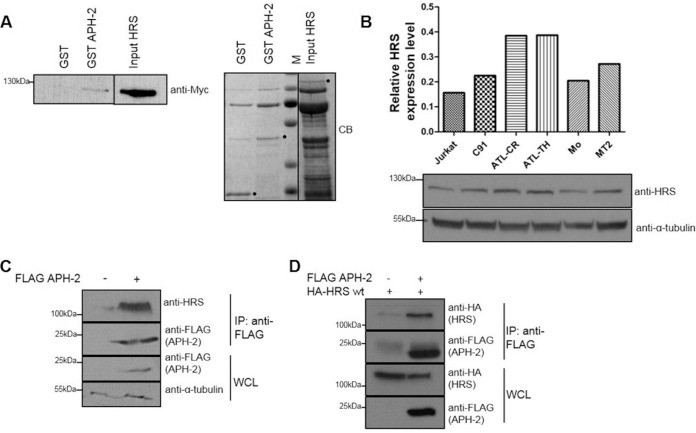
FIG 1.
APH-2 directly interacts with the ESCRT-0 subunit HRS. (A) GST pulldown assays were performed by incubating purified HRS-His-Myc with GST or GST-APH-2 immobilized on GST resin. Eluates were analyzed by immunoblotting using an anti-Myc antibody (left) and Coomassie blue (CB) staining (right). HRS input corresponds to 10% of the total HRS added to each pulldown. M indicates protein markers. Dots indicate purified GST, GST-APH-2, and HRS. (B) Expression levels of endogenous HRS in Jurkat cells, the HTLV-1-transformed cell lines MT2 and C91, the HTLV-2-transformed cell line Mo, and the ATL cell lines ATL-TH and ATL-CR were determined. Equal amounts of whole-cell lysates were analyzed by Western blotting using antibodies against HRS and α-tubulin. For the densitometric analysis, individual bands for HRS were quantified and normalized to the tubulin signal. (C and D) Coimmunoprecipitation assays were performed on lysates from HEK293T cells cotransfected with 10 μg of FLAG-APH-2 or empty plasmid (C) or 5 μg of FLAG-APH-2 together with either 5 μg of empty plasmid or 5 μg of HA-HRS expression plasmid (D). Proteins from whole-cell lysates (WCL) were immunoprecipitated using anti-FLAG M2 resin and were analyzed by Western blotting using anti-FLAG, anti-HRS, and anti-HA antibodies. IP, immunoprecipitation; wt, wild type.