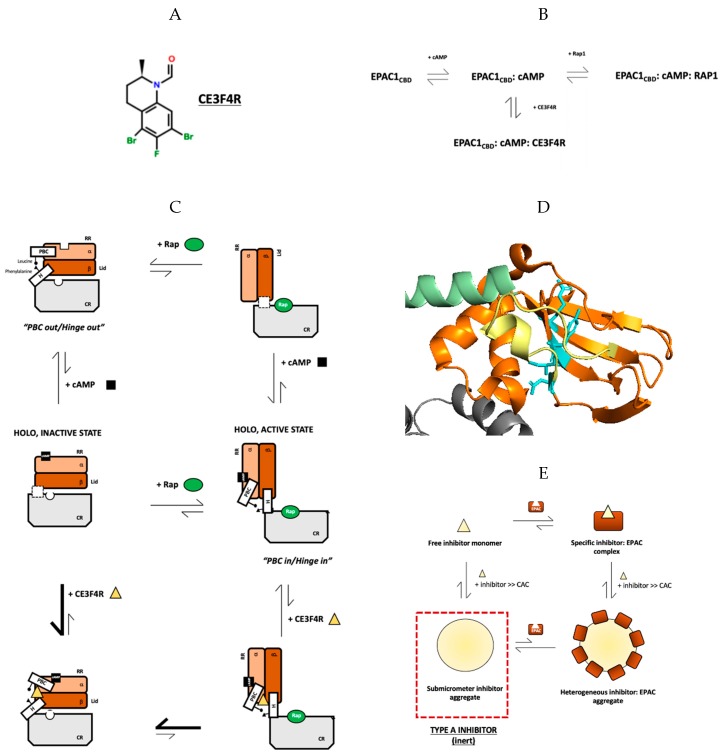
Figure 2.
Specific and non-specific interactions of EPAC1CBD and CE3F4R, a novel uncompetitive inhibitor. (A) The molecular structure of CE3F4R. (B) Schematic representing the uncompetitive mechanism of EPAC1 inhibition by CE3F4R. (C) Schematic summarizing the perturbation of the classic four-state thermodynamic cycle of EPAC activation by cAMP by CE3F4R binding, particularly highlighting the stabilization of the mixed holo inactive intermediate with the phosphate-binding cassette (PBC) in the active and hinge helix in the inactive conformation. Relative conformations of the PBC and hinge helix have not yet been elucidated in the holo, inactive and apo, active states and are thus not shown (D) Specific binding site of CE3F4R at the α/β subdomain interface of EPAC1 including residues Y242, I243, D267, and R294, as indicated in cyan, at the β-sheet facing the α-subdomain; the image shows homologous residues in EPAC2. Color scheme followed is consistent with Figure 1B. (E) Proposed thermodynamic cycle encompassing both specific enzyme:inhibitor binding as well as non-specific interactions between the two species as a result of colloidal aggregate formation; CE3F4R, as indicated on the figure, is a type-A inhibitor, forming inert aggregates that do not interact directly with the protein. Instead, they reduce overall inhibitory effect by acting as sinks for monomeric inhibitors (Figure adapted from Boulton, S.; Selvaratnam, R.; Ahmed, R.; Van, K.; Cheng, X.; Melacini, G. Mechanisms of specific versus nonspecific interactions of aggregation-prone inhibitors and attenuators. J. Med. Chem. 2019, 62, 5063–5079. Copyright (2019) American Chemical Society).