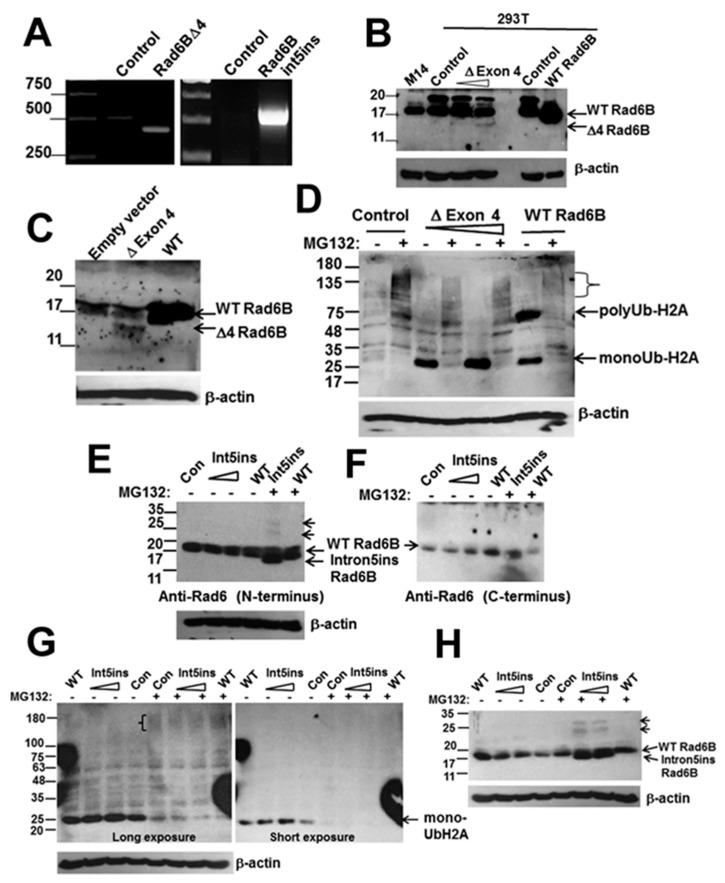
Figure 3.
The splice mutants RAD6BΔexon4 and RAD6Bintron5ins are catalytically active. (A) RT-PCR analysis of RAD6BΔexon4 and RAD6Bintron5ins expressions in 293T or COS7 transfected cells. (B) and (C), Western blot analysis of RAD6B using the C-terminus reactive antibody in control, wild-type (WT) RAD6B and RAD6BΔexon4 transfected 293T (B) and COS7 (C) cells. In (B), M14 cells were used as a melanoma control for biological validation of endogenous and exogenous WT RAD6B expressed in 293T cells. (D) In vivo ubiquitination activities of WT RAD6B and RAD6BΔexon4 were assessed in transfected COS7 cells with and without MG132 treatment by Western blotting with K119 ubiquityl histone H2A antibody. The positions of the mono- and polyubiquitinated H2A are indicated. The high molecular weight species of ubiquitinated histone H2A accumulating in MG132 treated control, RAD6BΔexon4 or WT RAD6B transfected cells are denoted by a bracket. (E) Western blot analysis of RAD6B using the N-terminus reactive antibody in control, WT RAD6B and RAD6B intron5ins transfected COS7 cells with and without MG132 treatment. Note that the 15 kDa intron5ins mutant is detected only in MG132 treated cells. Also note the presence of higher molecular weight RAD6B mutant bands in MG132 treated cells indicated by short arrows. (F) Re-probing of the stripped blot in (E) with RAD6 antibody specific for the C-terminus. Note that the nascent and higher molecular weight forms of RAD6Bintron5ins are not detected with the C-terminus reactive RAD6 antibody. (G) In vivo ubiquitination activity of RAD6Bintron5ins mutant in COS7 cells with and without MG132 treatment. High molecular weight or polyubiquitinated histone H2A detected in MG132 treated cells is indicted by a bracket. (H) The blot in (G) was stripped and re-probed with RAD6 antibody specific for the N-terminus and β-actin. Note the commensurate appearance of the RAD6B mutant and its mono- and di-ubiquitinated forms in MG132 treated cells.