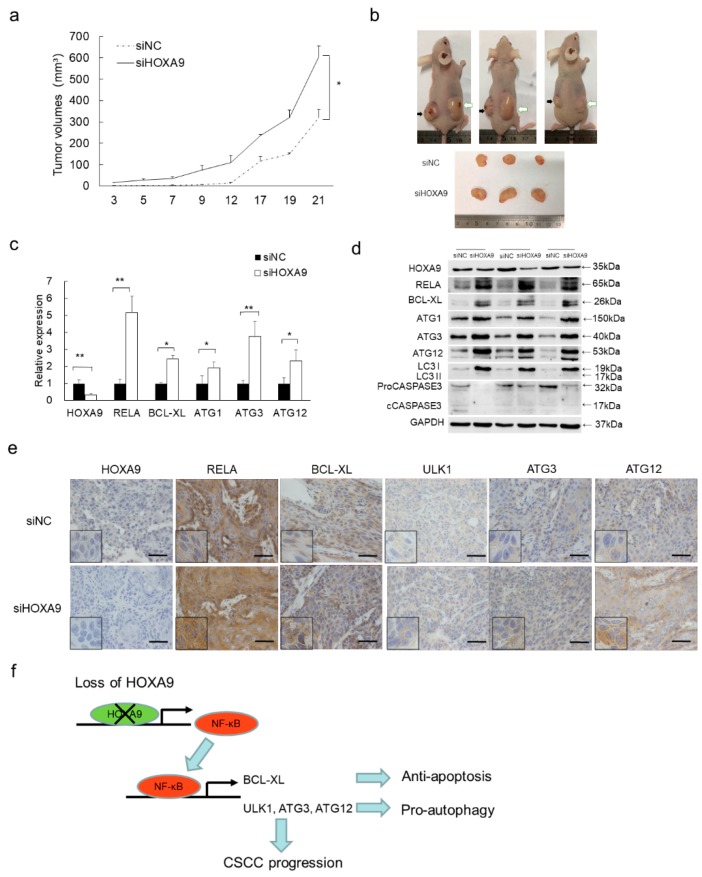
Figure 4.
Loss of HOXA9 inhibited apoptosis, promoted autophagy and tumor growth in vivo. (a) HOXA9 depletion enhances xenografts growth. Statistical data of tumor volumes represent the average of three independent experiments ± s.d, respectively. (b) Dissected xenografts from three sacrificed mice were imaged at the end of experiment. Black arrows point the siNC-treated xenografts while white arrows indicate siHOXA9-treated xenografts. (c) The expression of HOXA9, RELA, BCL-XL, ULK1, ATG3, and ATG12 was detected in the dissected xenografts by qRT-PCR. Statistical data of qRT-PCR represent the average of three independent experiments ± s.d. (d) The protein expression levels of HOXA9, RELA (p65), BCL-XL, ULK1, ATG3, and ATG12 was detected in xenografts after siHOXA9 treatment by western blot. (e) Histopathology analysis (IHC staining) of HOXA9, RELA (p65), BCL-XL, ULK1, ATG3, and ATG12 on tumor sections. Scale bar, 100 µm. (f) A model of the HOXA9-NF-κB regulatory axis in cSCC development. In cSCC tumors, loss of HOXA9 up-regulates NF-κB and its downstream anti-apoptotic gene BCL-XL and pro-autophagic genes of ULK1, ATG3, and ATG12, which contributes to the repressed apoptosis, enhanced autophagy and promotes cSCC progression. * p < 0.05, ** p < 0.01.