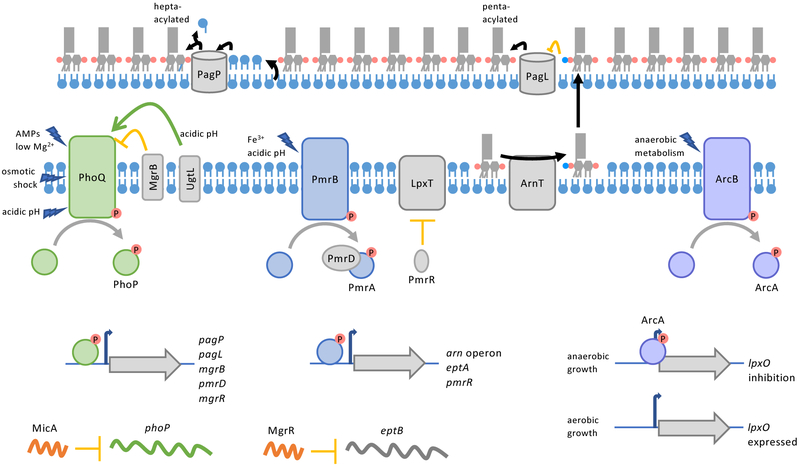
Figure 2: Regulation of LPS modifications.
(a) Regulation of LPS modification enzymes in Salmonella enterica subsp. enterica serovar Typhimurium. TCS PhoPQ, PmrAB, and ArcAB regulate genes that encode enzymes that alter the acylation (PagP, PagL), modify phosphates (aminoarabinose by ArnT and phosphoethanolamine by EptA), and hydroxylate an acyl chain (LpxO) of lipid A, respectively. PhoPQ also upregulates the protein PmrD which binds to and protects phosphorylated-PmrA, connecting these TCS. Small RNAs are connected to PhoPQ regulation; MicA inhibits translation of PhoP, and MgrR, when upregulated by PhoPQ, inhibits the gene eptB, encoding a core-oligosaccharide modifying phosphoethanolamine transferase. In addition, PhoPQ and PmrAB upregulate genes involved in negative feed-back loops for the respective TCS. PhoPQ upregulates MgrB that binds to and inhibits PhoQ. PmrAB upregulates the small protein PmrR and genes that for modifying lipid A with aminoarabinose (arn operon) and phosphoethanolamine (eptA). PmrR post-translationally inhibits the lipid A phosphotransferase LpxT. Decrease in LpxT activity and increased lipid A modification by ArnT and EptA alter the charge of the OM so that metal ions that activate PmrB are blocked from entering the cell. Finally, the activity of OM enzymes PagP and PagL are regulated by availability and modification of their substrates, respectively. When glycerophospholipids are mislocalized to the outer leaflet of the OM, PagP catalyzes the transfer of an acyl chain from donor glycerophospholipids to acceptor lipid A molecules. PagL deacylation of lipid A is inhibited by aminoarabinose modification of lipid A.