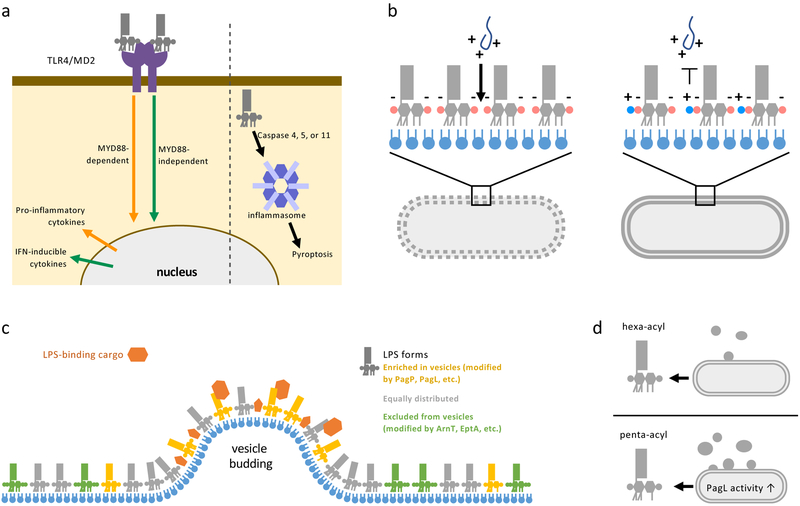
Figure 3: Consequences of LPS modifications.
(a) LPS can stimulate immune cell responses through recognition by surface receptors (left) or binding to the cytoplasmic inflammasome (right). TLR4/MD2 receptors on the surface of mammalian immune cells recognize lipid A and can activate two signaling pathways. The myeloid differentiation primary response protein 88 (MYD88)-dependent pathway upregulates proinflammatory cytokines that lead to inflammation and bacterial clearance. Alternatively, signaling through TIR domain-containing adaptor inducing IFNβ (TRIF), known as the MYD88-independent pathway, produces interferon (IFN) inducible cytokines that result in less inflammation, but are critical for adjuvanticity. Modifications of the phosphates and acyl chains of lipid A affect how well LPS is recognized by the TLR4/MD2 receptor and which signaling pathway is induced. Inflammasome recognition is mediates by caspases and lead to an inflammatory cell death pathway called pyroptosis. Modifications to acyl chains of LPS reduce stimulation of murine inflammasome response but does not affect stimulation of human cell-lines inflammasomes. (b) Cationic antimicrobial peptides (AMPs) produced by host immune cells or used as antibiotics (such as Polymyxin B and Colistin) to treat infectious bacteria act by first forming charge-charge interactions with the highly negatively-charged OM. AMPs then perforate the OM followed by the IM, leading to lysis of bacterial cells. LPS modification that reduce the negative charge or alter the acyl chains of lipid A provide resistance against AMPs by charge repulsion or decreasing the fluidity of the OM. (c) Gram-negative bacteria release vesicles that bud from the OM called outer membrane vesicle (OMVs). When the LPS in the OM and OMVs released are compared, certain chemical forms of LPS are enriched, equally distributed, or excluded (colored gold, grey, and green respectively) in OMVs. Some cargo proteins (orange) associated with OMVs such as heat-labile enterotoxin in enterotoxigenic E. coli, are recruited through specific interactions with LPS. Allowing the selective recruiting and secretion of certain proteins in OMVs. (d) Certain chemical forms of LPS, such as penta-acylated LPS produced by PagL activity, increase the size and number of OMVs released by bacteria, indicating that LPS modification can stimulate OMV formation.