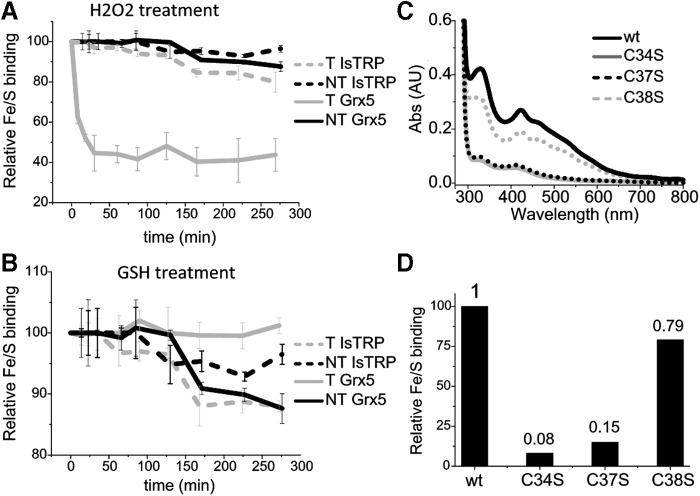
FIG. 3.
IsTRP and Grx5 Fe/S-binding properties. (A, B) Kinetics for the disassembly of holo-IsTRP and holo-Grx5 (NT) and upon treatment (T) with 10 mM of H2O2 or 3 mM of GSH. The loss of the Fe/S was recorded by following the decrease in absorbance at 320 nm. Error bars correspond to standard deviations of three replicates. (C, D) The Fe/S coordination ability of wild-type, Cys38Ser, Cys37Ser, and Cys34Ser mutants was analyzed by measuring the absorbance at 320 nm of the copurified holoproteins. Protein concentration was between 300 and 200 μM. A representative UV-visible spectrum (normalized to protein concentration) of each protein species is shown (C) and the corresponding 320/280 nm ratio relative to the binding of the wild-type protein is shown (D). GSH, glutathione.