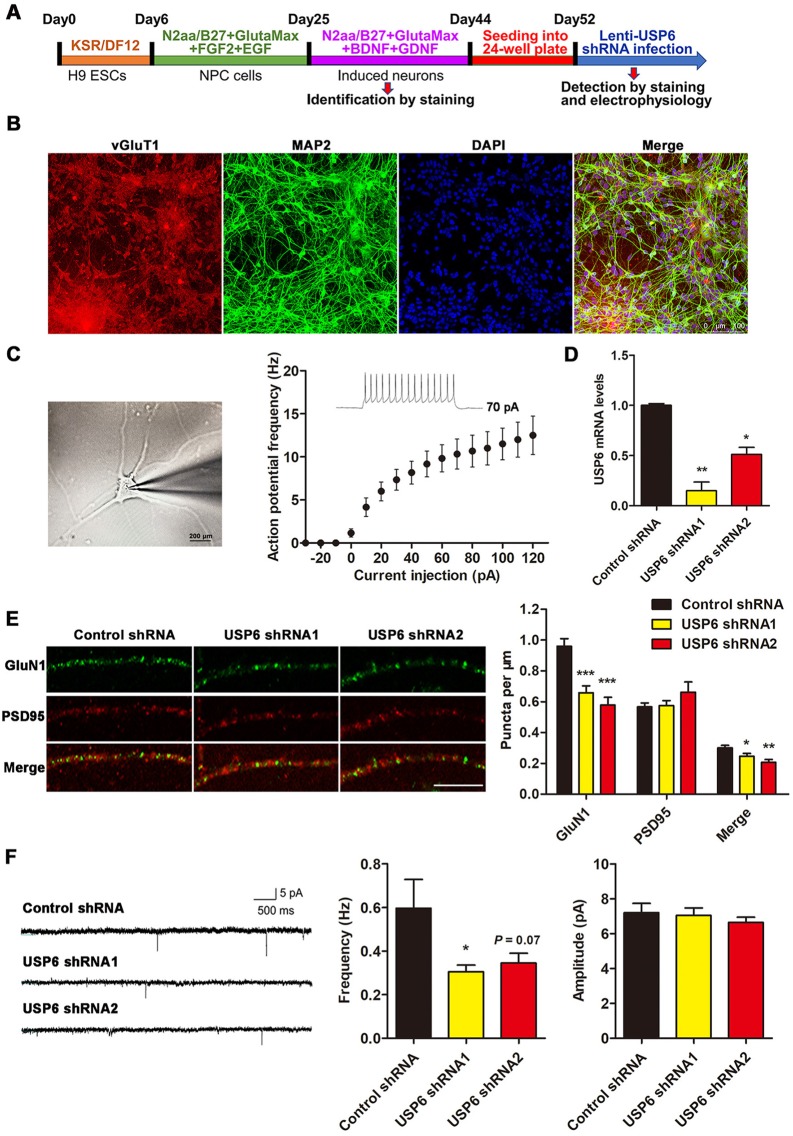
Fig 8. USP6 depletion reduces GluN1 expression in ESC-derived human excitatory neurons.
(A) Schematic diagram depicting the differentiation timescale and experimental timeline in ESC-derived human excitatory neurons. (B) Immunostaining for vGluT1 and MAP2 at day 44 of differentiation. (C) Action potential at day 60 of differentiation in ESC-derived human excitatory neurons. (D) Knockdown efficiency of USP6 shRNAs in induced human excitatory neurons as quantified by qRT-PCR analysis. Data represent means ± SEM. n = 3. **P < 0.01, ***P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc analysis. (E) Immunostaining for GluN1 and PSD95 in induced human excitatory neurons transduced with lentiviral USP6 shRNA. Quantification of GluN1 puncta: control shRNA (n = 43 neurites from 36 neurons), USP6 shRNA1 (n = 36 neurites from 19 neurons), and USP6 shRNA2 (n = 21 neurites from 15 neurons); quantification of PSD95 puncta: control shRNA (n = 61 neurites from 44 neurons), USP6 shRNA1 (n = 29 neurites from 23 neurons), and USP6 shRNA2 (n = 21 neurites from 15 neurons). The data represent means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc analysis. (F) Representative NMDA mEPSC recordings in induced human excitatory neurons infected with USP6 shRNA lentivirus. Data represent means ± SEM. n = 3. *P < 0.05 as determined by one-way ANOVA with Tukey’s post hoc analysis. The underlying data for this figure can be found in S1 Data. B27, B-27 serum-free supplement; BDNF, brain-derived neurotrophic factor; EGF, epidermal growth factor receptor; ESC, embryonic stem cell; FGF2, fibroblast growth factor 2; GDNF, glial cell–derived neurotrophic factor; Glu, glutamate ionotropic receptor; MAP2, microtubule-associated protein 2; mEPSC, miniature excitatory postsynaptic current; N2aa, DMEM-F12 medium with N2-supplement and ascorbic acid; NMDA, N-methyl-D-aspartate; NPC, neural progenitor cell; PSD, postsynaptic density; qRT-PCR, quantitative reverse transcription PCR; shRNA, short hairpin RNA; USP, ubiquitin-specific protease; vGluT1, vesicular glutamate transporter.