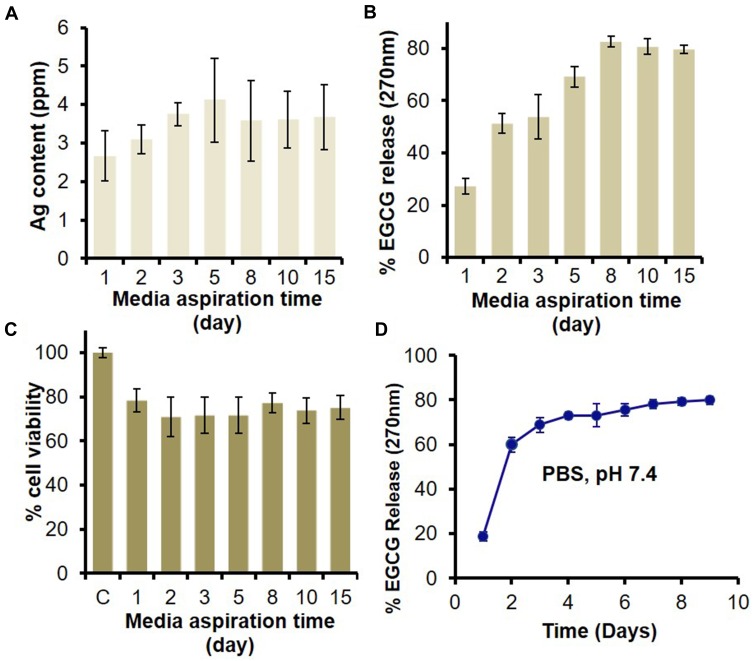
Figure 4.
In vitro release of Ag and EGCG from HG-Ag-EGCG and their effect on biocompatibility. HG-Ag-EGCG was placed in DMEM culture medium to unloose its content. The media was aspirated at different time points (1, 3, 5, 8, 10, 15 days) and subjected to analyses. (A) AAS estimation of Ag content with a maximum concentration of 4.1 ppm. (B) UV analysis for EGCG release shows sustained release profile in aspirated media and ~80% of EGCG was released in 8th day which remains constant afterward. (C) MTT assay for cell viability on aspirated media treated normal skin MSC P5 cells indicates good biocompatibility of Hg-Ag-EGCG. (D) EGCG release kinetics from final formulation (Hg-Ag-EGCG) at a different time interval in PBS; pH 7.4 (mean ± SD, n = 3).