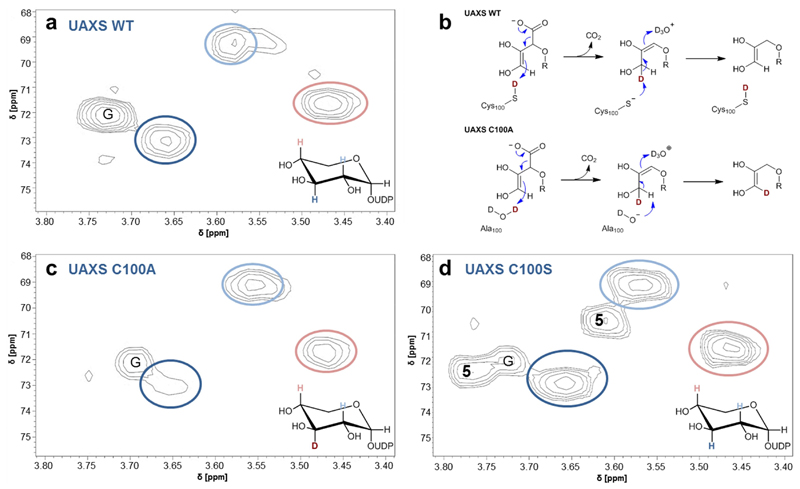
Fig. 4. Deuterium incorporation at C3’ during conversion of UDP-GlcA (2) by wild-type and C100A and C100S forms of UAXS.
Panels (a), (b) and (d) show results of hetero-nuclear single quantum coherence (HSQC) experiments, analyzing the UDP-xylose (3) isolated from reactions in D2O (pD 7.0) of wild-type UAXS (a), C100A variant (c) and C100S variant (d). Relevant hydrogens at carbons 2’, 3’ and 4’ are highlighted in color and are indicated in the spectra with correspondingly colored circles. G = signal derived from traces of glycerol. 5 = signals derived from UDP-4-keto-xylose (C3-H at ∼3.78 ppm; C2-H at ∼3.61 ppm). Panel (b) shows protonation and deprotonation events during the decarboxylation, leading to deuterium incorporation at C3’ in the reaction of the C100A variant but not in the reaction of the wild-type enzyme. Note: in all enzymatic reactions, the C5’ in UDP-xylose (3) is partly deuterated (50%) from solvent, as expected from the proposed mechanism (panel b).