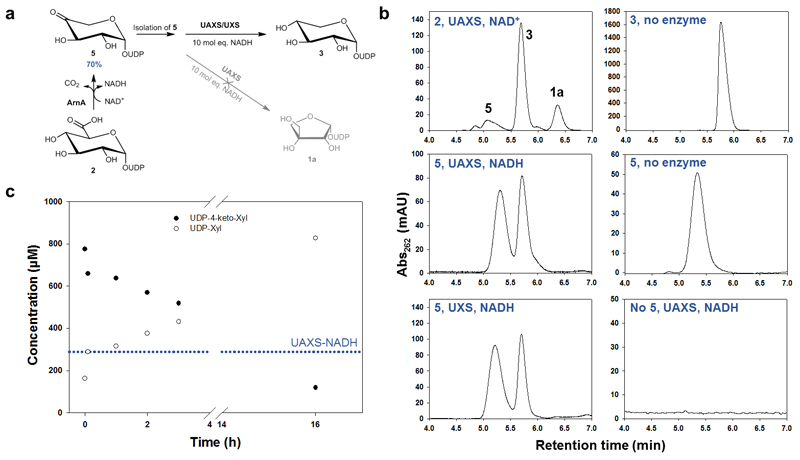
Fig. 5. Reaction of UAXS with UDP-4-keto-xylose (5) to study ring opening and reduction by the enzyme.
Panel (a) shows the different steps of the experiment. The UDP-4-keto-xylose (5) was synthesized from UDP-GlcA (2) using the enzyme ArnA. The isolated compound 5 was then subjected to conversion by UAXS in the presence of excess of NADH. As a reference, the reaction was also performed with UXS. Panel (b) shows HPLC traces from the conversions of UDP-4-keto-xylose (5) with wild-type UAXS and human UXS as well as the most relevant controls. Panel (c) shows a time course for the reduction of UDP-4-keto-xylose (5) by wild-type UAXS. The blue dotted line indicates the concentration of enzyme-bound NADH in UAXS (here ∼60% of 412 μM enzyme).