Fig. 1.
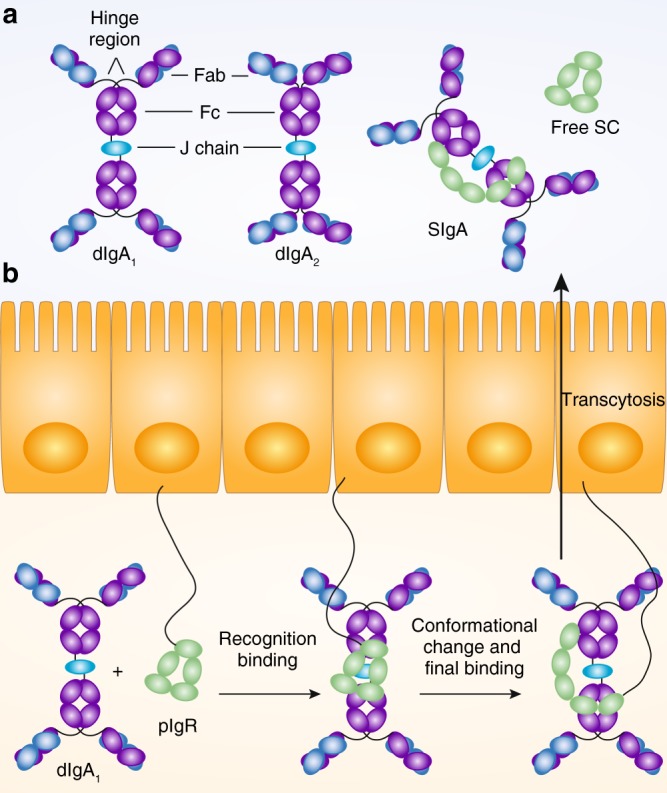
Secretory IgA is formed by the combined function of plasma cells producing multimeric IgA and epithelial cells expressing pIgR a Schematic diagrams illustrating the structure of human dimeric IgA1, human dimeric IgA2, human secretory IgA1, and the free secretory component (SC, which is a cleavage product of pIgR). Both, human IgA1 and IgA2 show the canonical antibody structure of two heavy and two light chains building Fab and Fc portions of the antibody. Human IgA1 is characterized by an extended hinge region linking the Fab and Fc part. In dimeric IgA, two antibody monomers are covalently bound through disulfide bonds to the J chain. Secretory component covalently bound to IgA differs in its conformation from free SC. Consequently, free SC and bound SC might have different microbiota binding capacity. b Transcytosis of pIgR/dIgA complexes results from initial recognition binding, conformational changes, and final binding before the complex becomes transcytosed. Following transcytosis, free SC, and SIgA are released into the gut lumen (here depicted for human IgA1). Illustrations adapted from refs.4,68
