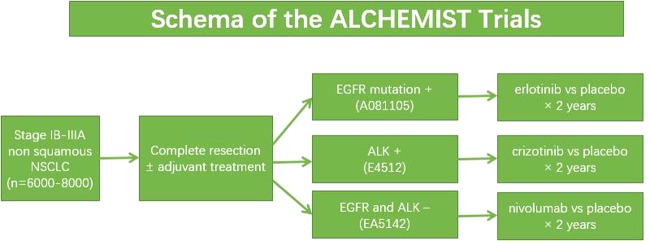
Fig. 4. Schematic of the ALCHEMIST trial.
8000 patients are planned to be screened through this trial to facilitate accrual to three substudies: ALCHEMISTEGFR (A081105; NCT02193282), ALCHEMIST-ALK (E4512; NCT02201992), and ALCHEMIST-nivo (EA5142; NCT02595944). In this trial, patients will have first undergone resection and completed standard adjuvant therapy. The patients with EGFR-mutated NSCLC will be randomized 1:1 to either erlotinib or placebo. The patients with ALK-rearranged NSCLC will be randomized 1:1 to crizotinib or placebo. The patients who are negative for EGFR or ALK mutations will be randomized to adjuvant nivolumab or observation for 1 year.