Figure 5.
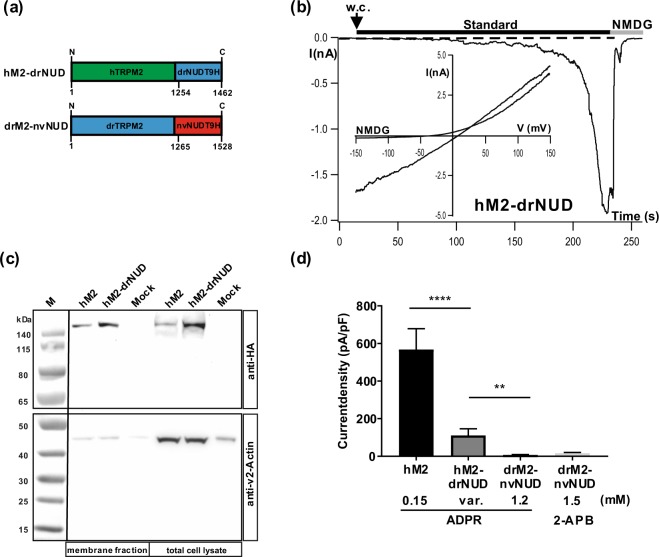
hTRPM2 is basically functional with the NUDT9H domain of drTRPM2. (a) Sketch of the chimeras hTRPM2-drNUD and drTRPM2-nvNUD where the NUDT9H-domains between the TRPM2 orthologues of human, zebrafish and sea anemone are exchanged as indicated. For the exact procedure see Methods. (b) Example for a successful stimulation of a HEK-293 cell expressing hTRPM2-drNUD with ADPR during whole-cell patch-clamp analysis. ADPR (1.2 mM) was infused into the cell through the patch pipette together with 1 µM Ca2+. Current develops gradually after reaching whole-cell configuration (w.c.). (c) Cell surface expression of wild-type hTRPM2 and of the chimera hTRPM2-drNUD was analyzed by biotinylation and subsequent reducing 4–12% SDS-PAGE. Mock-transfected cells were used as negative control. One gel was loaded with the eluted membrane proteins, another gel was loaded with the corresponding total cell lysates. PVDF membranes were cut between the marker bands for 50 and 65 kDa. The upper part was incubated with anti-HA, the lower part with anti-β-actin antibody. Different exposure times were needed due to differences in signal intensity between HA and β-actin. Wild-type hTRPM2 as well as the chimera hTRPM2-drNUD were detected in the Avidin-bound fraction representing the pool of biotinylated surface expressed proteins. Reduced β-actin staining in the membrane fraction indicates biotinylation of cytosolic proteins in damaged cells. Three independent experiments gave similar results. (d) Summary of the experiments investigating the channel activity of hTRPM2-drNUD and drTRPM2-nvNUD. Wild-type hTRPM2 was used as the positive control. For hTRPM2-drNUD only responders were considered which were obtained at various (var.) concentrations of ADPR. All data are presented as mean ± s.e.m. Differences are significant at **(P < 0.01) ****(P < 0.0001), evaluated with one-way ANOVA and the Bonferroni correction, n = 3–9. n.s., not significant.