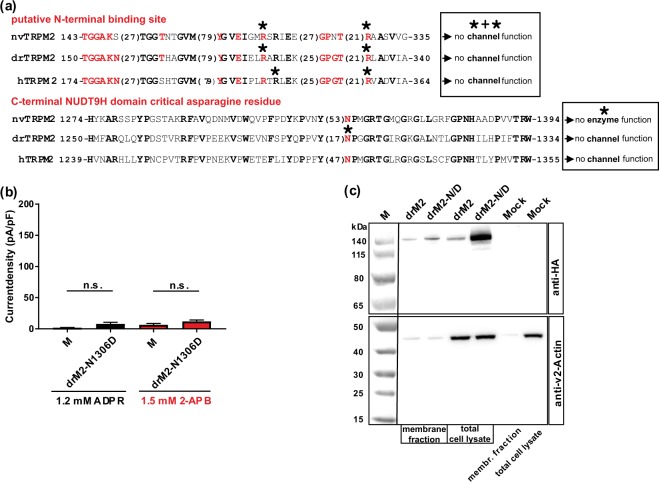
Figure 6.
Functional importance of a highly conserved asparagine residue in NUDT9H. (a) Partial sequences of the TRPM2 orthologues of human, sea anemone and zebrafish encompassing the putative N-terminal ADPR-binding pocket or the N-terminal part of the NUDT9H domain (as indicated). The numbers given at the beginning and at the end of each sequence indicate the exact position within the corresponding open reading frame. Numbers in brackets specify the lengths of spacer sequences. Conserved amino acid residues are given in bold. The amino acid residues forming the novel ADPR-binding pocket as well as the critical and highly conserved asparagine residue of NUDT9H are highlighted in red. Residues which have been mutated in this study are marked with an asterisk. The two text boxes summarize functional effects of these mutations. (b) Summary of the experiments investigating the channel activity of a mutant of drTRPM2 where the corresponding asparagine of the NUDT9H domain was changed to aspartate (mutation N1306D). Stimulations were performed with ADPR or with 2-APB as indicated. All data are presented as mean ± s.e.m. Statistical analysis was performed with an unpaired Student’s t-test, n = 4–8. n.s., not significant. For comparison note the scale of the ordinates of Figs. 1d, 4d. (c) Comparison of cell surface expression of drTRPM2-N1306D (abbreviated as drM2-N/D) and wild-type drTRPM2 as indicated. Analysis was performed as described in Fig. 5c. Wild-type drTRPM2 as well as the mutant drTRPM2-N1306D were detected in the Avidin-bound fraction representing the pool of biotinylated surface expressed proteins. Reduced β-actin staining in the membrane fraction indicates biotinylation of cytosolic proteins in damaged cells. Three independent experiments gave similar results.