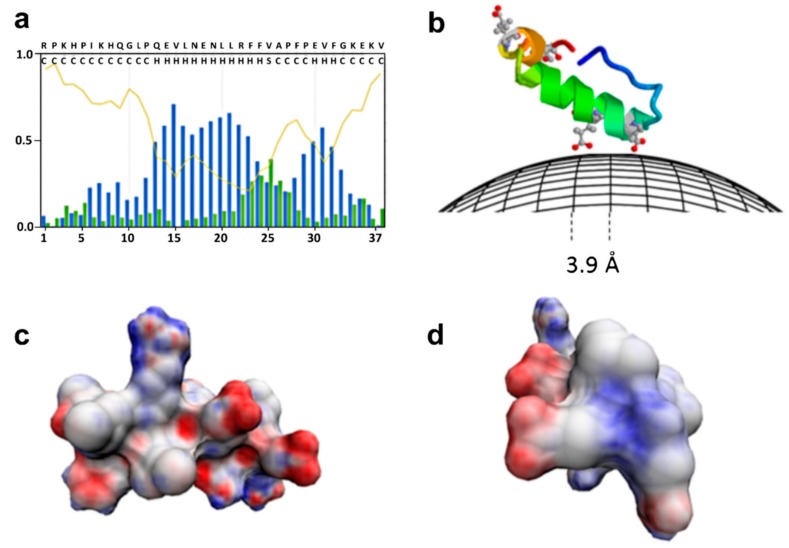
Figure 4.
Binding of a milk peptide (αs1-casein 1-37)) on the surface of BIONs. (a) Statistical populations of ordered and disordered regions for the peptide according to the prediction of the s2D method. Coil curve describes the prediction of the probability to be unstructured and flexible. Blue and green bars represent the prediction of the statistical population for the helix and strand conformations; (b) graphical sketch of the peptide binding on BIONs where structured parts of the peptide are represented by helical models. The peptide structure was created by running QUARK algorithm; (c) and (d) helical model of the portion of the peptide predicted to be in helix according to s2D, two different views. The negatively charged surface is highlighted in red and computed with a Poisson–Boltzmann electrostatics calculations by 3D-HM. Reproduced from [69], with permission from Springer Nature, 2019.