Figure 3.
Enzymatically Active HLCs Are Generated from PGPC iPSCs
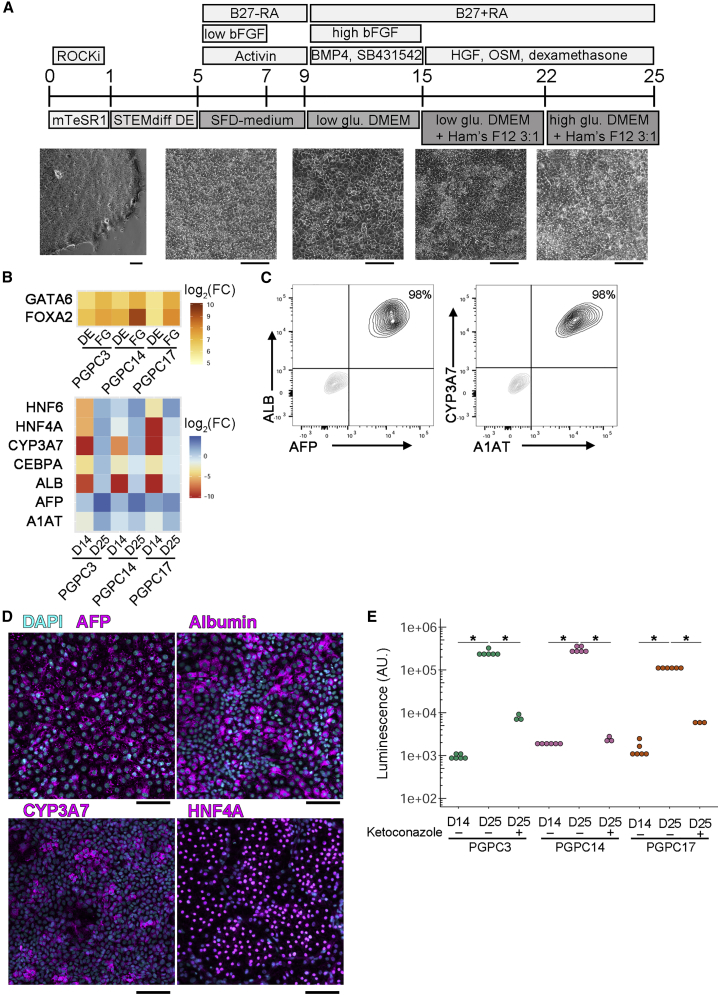
(A) Hepatocyte-like cell differentiation scheme. iPSCs were dissociated to single cells and maintained in ROCK inhibitor for 24 h to support survival. From D1 to D4 cells are transferred to STEMdiff definitive endoderm (DE) differentiation kit. From D5 to D9 cells were switched to serum-free differentiation (SFD)-based medium with activin A for 4 days and basic fibroblast growth factor (bFGF) for 2 days adding B27 without retinoic acid (RA). Medium was changed every other day, then on D9 cells were switched to low glucose DMEM supplemented with SB-431542 and bone morphogenic protein (BMP4) from day 9 to day 15. bFGF was added back to the medium during D9 to D15 while B27 with RA was supplemented from D9 to D25 with medium changed every other day. From D15 to D25 hepatocyte growth factor (HGF), dexamethasone, and oncostatin M (OSM) were added to culture medium. From D15 to D21, cells were cultured in a mixture of low glucose DMEM/Ham's F12 (3:1) medium, and at D22 cells were cultured in a mixture of high glucose DMEM/Ham's F12 (3:1) medium. Bright-field images highlight morphology changes during differentiation. Scale bars represent 100 μm.
(B) Heatmaps indicating log2 fold change of marker gene expression normalized to iPSCs (top) or to fetal liver (bottom) (independent experiments = 3).
(C) Dissociated D25 PGPC14 HLCs were labeled with anti-ALB and anti-AFP (left) or anti-alpha-1-AT and anti-CYP3A7 (right) and subjected to flow cytometry (independent experiments = 3).
(D) Representative immunocytochemistry images of D25 PGPC14 HLCs labeled with DAPI and anti-AFP, anti-albumin, anti-CYP3A7, or anti-HNF4A (independent experiments = 3). Scale bars represent 100 μm. Color channels were independently altered to adjust contrast for publication.
(E) Log scale plot of luminescence of each cell line measuring P450 enzymatic activity assayed at D14 and D25 with or without ketoconazole as an inhibitor (independent experiments n = 3; technical replicates for untreated samples = 2, treated samples = 1). Statistical significance was determined by Dunn's test between all samples and ∗ indicate pairs where p < 0.05.