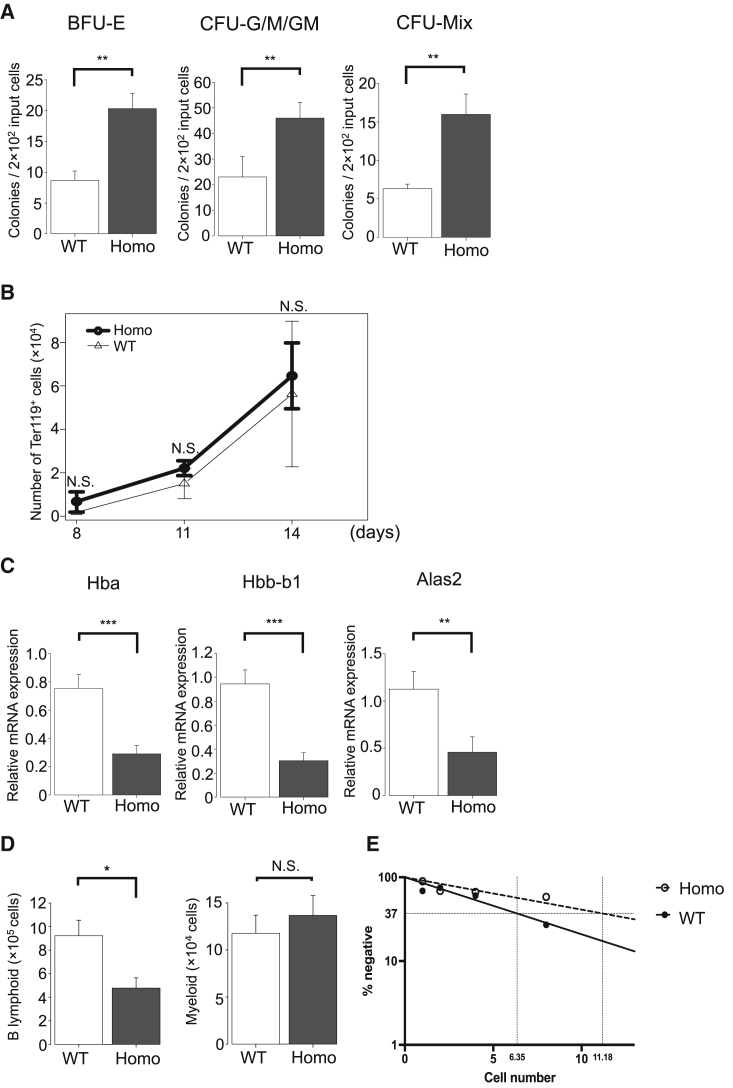
Figure 3.
ESAM-Null HSCs Exhibited Functional Disruption of Differentiation in Culture
(A) The sorted LSK CD48– cells of E14.5 WT or ESAM Homo KO littermates were cultured in methylcellulose medium. The number of granulocyte colony-forming units (CFU-G), macrophage colony-forming units (CFU-M), granulocyte-macrophage colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E), or mixed erythroid-myeloid colony-forming units (CFU-Mix) are shown (n = 3, each group).
(B) The sorted LSK CD48– cells of E14.5 WT or ESAM Homo KO littermates were cocultured in BM stromal cell lines (MS-5), under appropriate conditions to produce erythroid cells. After 8, 11, and 14 days of culture, cells were collected and analyzed by fluorescence-activated cell sorting (FACS). The numbers of Ter119+ erythroid cells are shown over time (n = 4, each group).
(C) The mRNA expression levels of Hba, Hbb-b1, and Alas2 in the BFU-E colonies analyzed by qRT-PCR (n = 15, each group).
(D) Sorted LSK cells of E14.5 WT or ESAM Homo KO littermates (100 cells/well) were cocultured with MS-5 under conditions to produce B lymphoid and myeloid cells. After 10 days of culture, cells were collected and analyzed by FACS. The numbers of CD19+ B lymphoid cells and Mac1+ myeloid cells are shown (n = 4, each group).
(E) FL LSK CD48– HSCs collected at E14.5 from WT or ESAM Homo KO fetuses were subjected to limiting dilution analyses in the MS-5 coculture system. Input cell numbers corresponding to 37% negative value are shown in rectangles.
Data are shown as means ± SEM. Statistically significant differences are represented by asterisks: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.