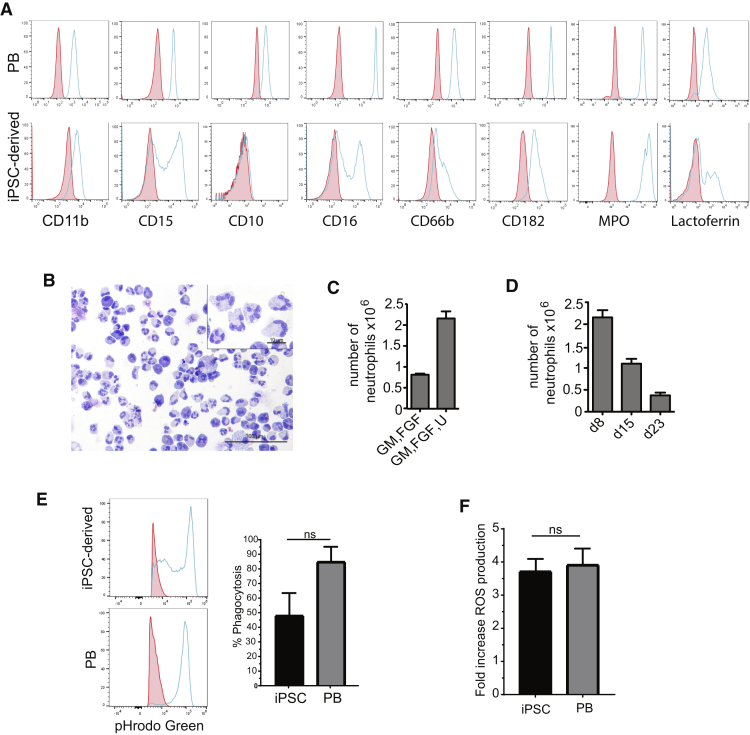
Figure 3.
Induction of Neutrophil Formation from Myeloid Progenitors
(A) Flow cytometric analysis of generated neutrophils. Plots show unstained control (red) and specific antibody (blue) histograms.
(B) Cytospin showing the morphology of the generated neutrophils. Scale bars, 100 μm and 10 μm (insert).
(C) Neutrophil yields from 106 myeloid progenitors that were cultured with or without UM171 and collected on day 8 after ETV2 mmRNA transfection. Bars show mean ± SE for 3 (GM, FGF, and U) and 2 (GM and FGF) independent experiments.
(D) Total number of neutrophils obtained from 106 myeloid progenitors that were cultured with UM171 and collected at different days of after ETV2 mmRNA transfection. Bars show mean ± SE for 2 independent experiments.
(E) Phagocytosis of pHrodo Green E. coli particles by neutrophils generated from IISH2i-BM9 hiPSCs. Solid red peaks on flow graphs are control cells incubated on ice with bio-particles, blue traces are cells containing acidified, fluorescent E. coli bio-particles from 37°C incubation. Bar graph is from 3 independent experiments showing percent of cells from 37°C incubation with phagocytosed acidified E. coli bio-particles. Bars show mean ± SE. Difference between iPSC and primary neutrophils is not statistically significant (p = 0.3134) as determined by unpaired t test.
(F) Reactive oxygen species production of hiPSC-derived neutrophils compared with primary blood neutrophils at 90 min. Bar graph is from 3 independent experiments showing fold increase of 50 ng/mL PMA-treated cells over control-treated cells. Bars show mean ± SE. Difference between hiPSC versus primary neutrophils is not statistically significant (p = 0.7522) as determined by unpaired t test.
See also Figure S2.